An orally bioavailable SARS-CoV-2 main protease inhibitor exhibits improved affinity and reduced sensitivity to mutations
- PMID: 38478629
- PMCID: PMC11193659
- DOI: 10.1126/scitranslmed.adi0979
An orally bioavailable SARS-CoV-2 main protease inhibitor exhibits improved affinity and reduced sensitivity to mutations
Abstract
Inhibitors of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) main protease (Mpro) such as nirmatrelvir (NTV) and ensitrelvir (ETV) have proven effective in reducing the severity of COVID-19, but the presence of resistance-conferring mutations in sequenced viral genomes raises concerns about future drug resistance. Second-generation oral drugs that retain function against these mutants are thus urgently needed. We hypothesized that the covalent hepatitis C virus protease inhibitor boceprevir (BPV) could serve as the basis for orally bioavailable drugs that inhibit SARS-CoV-2 Mpro more efficiently than existing drugs. Performing structure-guided modifications of BPV, we developed a picomolar-affinity inhibitor, ML2006a4, with antiviral activity, oral pharmacokinetics, and therapeutic efficacy similar or superior to those of NTV. A crucial feature of ML2006a4 is a derivatization of the ketoamide reactive group that improves cell permeability and oral bioavailability. Last, ML2006a4 was found to be less sensitive to several mutations that cause resistance to NTV or ETV and occur in the natural SARS-CoV-2 population. Thus, anticipatory design can preemptively address potential resistance mechanisms to expand future treatment options against coronavirus variants.
Conflict of interest statement
WO 2021/226546 A1 PROTEASE INHIBITORS FOR TREATMENT OR PREVENTION OF CORONAVIRUS DISEASE. MZL, MW, and YS are co-inventors on patent application WO 2023/114516 A2 CELL-PERMEANT INHIBITORS OF VIRAL CYSTEINE PROTEASES. These patent applications describe compounds developed in this study.
Figures


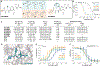

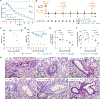

Similar articles
-
Structural basis for varying drug resistance of SARS-CoV-2 Mpro E166 variants.mBio. 2025 Jul 9;16(7):e0262424. doi: 10.1128/mbio.02624-24. Epub 2025 Jun 2. mBio. 2025. PMID: 40454888 Free PMC article.
-
Replication capacity and susceptibility of nirmatrelvir-resistant mutants to next-generation Mpro inhibitors in a SARS-CoV-2 replicon system.Antiviral Res. 2024 Nov;231:106022. doi: 10.1016/j.antiviral.2024.106022. Epub 2024 Oct 17. Antiviral Res. 2024. PMID: 39424074
-
AI-driven covalent drug design strategies targeting main protease (mpro) against SARS-CoV-2: structural insights and molecular mechanisms.J Biomol Struct Dyn. 2025 Jul;43(11):5436-5464. doi: 10.1080/07391102.2024.2308769. Epub 2024 Jan 29. J Biomol Struct Dyn. 2025. PMID: 38287509 Review.
-
SARS-CoV-2 Mpro inhibitor identification using a cellular gain-of-signal assay for high-throughput screening.SLAS Discov. 2024 Sep;29(6):100181. doi: 10.1016/j.slasd.2024.100181. Epub 2024 Aug 22. SLAS Discov. 2024. PMID: 39173830 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
Cited by
-
Structural basis for varying drug resistance of SARS-CoV-2 Mpro E166 variants.mBio. 2025 Jul 9;16(7):e0262424. doi: 10.1128/mbio.02624-24. Epub 2025 Jun 2. mBio. 2025. PMID: 40454888 Free PMC article.
-
Structure-based discovery of highly bioavailable, covalent, broad-spectrum coronavirus MPro inhibitors with potent in vivo efficacy.Sci Adv. 2025 Apr 25;11(17):eadt7836. doi: 10.1126/sciadv.adt7836. Epub 2025 Apr 23. Sci Adv. 2025. PMID: 40267184 Free PMC article.
-
Structure-Based Optimization of Pyridone α-Ketoamides as Inhibitors of the SARS-CoV-2 Main Protease.J Med Chem. 2025 Feb 13;68(3):2920-2941. doi: 10.1021/acs.jmedchem.4c02172. Epub 2025 Jan 16. J Med Chem. 2025. PMID: 39817813 Free PMC article.
-
On the origins of SARS-CoV-2 main protease inhibitors.RSC Med Chem. 2023 Oct 13;15(1):81-118. doi: 10.1039/d3md00493g. eCollection 2024 Jan 25. RSC Med Chem. 2023. PMID: 38283212 Free PMC article. Review.
-
Understanding emerging and re-emerging viruses to facilitate pandemic preparedness.Nat Microbiol. 2024 Sep;9(9):2208-2211. doi: 10.1038/s41564-024-01789-5. Nat Microbiol. 2024. PMID: 39198691 No abstract available.
References
-
- WHO, “COVID-19 Weekly Epidemiological Update - 29 September 2023” (2023).
-
- Tenforde MW, Self WH, Gaglani M, Ginde AA, Douin DJ, Talbot HK, Casey JD, Mohr NM, Zepeski A, McNeal T, Ghamande S, Gibbs KW, Files DC, Hager DN, Shehu A, Prekker ME, Frosch AE, Gong MN, Mohamed A, Johnson NJ, Srinivasan V, Steingrub JS, Peltan ID, Brown SM, Martin ET, Monto AS, Khan A, Hough CL, Busse LW, Duggal A, Wilson JG, Qadir N, Chang SY, Mallow C, Rivas C, Babcock HM, Kwon JH, Exline MC, Botros M, Lauring AS, Shapiro NI, Halasa N, Chappell JD, Grijalva CG, Rice TW, Jones ID, Stubblefield WB, Baughman A, Womack KN, Rhoads JP, Lindsell CJ, Hart KW, Zhu Y, Adams K, Surie D, McMorrow ML, Patel MM, Effectiveness of mRNA Vaccination in Preventing COVID-19–Associated Invasive Mechanical Ventilation and Death — United States, March 2021–January 2022. MMWR Morb. Mortal. Wkly. Rep. 71, 459–465 (2022). - PMC - PubMed
-
- Nyberg T, Ferguson NM, Nash SG, Webster HH, Flaxman S, Andrews N, Hinsley W, Bernal JL, Kall M, Bhatt S, Blomquist P, Zaidi A, Volz E, Aziz NA, Harman K, Funk S, Abbott S, Lopez Bernal J, Abdul Aziz N, Hope R, Charlett A, Chand M, Ghani AC, Seaman SR, Dabrera G, De Angelis D, Presanis AM, Thelwall S, Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. The Lancet 399, 1303–1312 (2022). - PMC - PubMed
-
- Deng X, St SE. John HL. Osswald A. O’Brien BS. Banach K Sleeman AK. Ghosh AD. Mesecar SC. Baker, Coronaviruses Resistant to a 3C-Like Protease Inhibitor Are Attenuated for Replication and Pathogenesis, Revealing a Low Genetic Barrier but High Fitness Cost of Resistance. J. Virol 88, 11886–11898 (2014). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

