Overlaid Lateral Flow Immunoassay for the Simultaneous Detection of Two Variant-Specific SARS-CoV-2 Neutralizing Antibodies
- PMID: 38478766
- PMCID: PMC11270523
- DOI: 10.1021/acs.analchem.3c05144
Overlaid Lateral Flow Immunoassay for the Simultaneous Detection of Two Variant-Specific SARS-CoV-2 Neutralizing Antibodies
Abstract
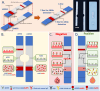
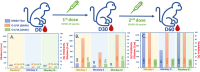
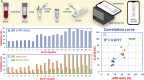
COVID-19 vaccines have been provided to the general public to build immunity since the 2019 coronavirus pandemic. Once vaccinated, SARS-CoV-2 neutralizing antibodies (NAbs-COVID-19) are needed for excellent protection against COVID-19. However, monitoring NAbs-COVID-19 is complicated and requires hospital visits. Moreover, the resulting NAbs-COVID-19 are effective against different strains of COVID-19 depending on the type of vaccine received. Here, an overlaid lateral flow immunoassay (O-LFIA) was developed for the simultaneous detection of two NAbs-COVID-19 against different virus strains, Delta and Omicron. The O-LFIA was visualized with two T-lines with a single device using competition between the free antigen and the antigen-binding antibody. Angiotensin-converting enzyme 2 (ACE2) immobilized on the T-line binds to the antigen remaining after antibody binding. Under the optimum conditions, the proposed device exhibited 50% inhibition concentrations (IC50 values) of 45.1 and 53.6 ng/mL for the Delta and Omicron variants, respectively. Additionally, the proposed platform was applied to real-world samples of animal and human serum, and the developed immunoassay provided results that were in good agreement with those obtained with the standard method. In conclusion, this developed O-LFIA can be used as an alternative method to detect NAbs-COVID-19 and can be enabled for future advancements toward commercialization.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Development of receptor binding domain-based double-antigen sandwich lateral flow immunoassay for the detection and evaluation of SARS-CoV-2 neutralizing antibody in clinical sera samples compared with the conventional virus neutralization test.Talanta. 2023 Apr 1;255:124200. doi: 10.1016/j.talanta.2022.124200. Epub 2022 Dec 21. Talanta. 2023. PMID: 36565525 Free PMC article.
-
Fluorescence-Quenching Lateral Flow Immunoassay for "Turn-On" and Sensitive Detection of Anti-SARS-Cov-2 Neutralizing Antibodies in Human Serum.Adv Sci (Weinh). 2024 Jan;11(4):e2305774. doi: 10.1002/advs.202305774. Epub 2023 Nov 30. Adv Sci (Weinh). 2024. PMID: 38032112 Free PMC article.
-
The instantly blocking-based fluorescent immunochromatographic assay for the detection of SARS-CoV-2 neutralizing antibody.Front Cell Infect Microbiol. 2023 Sep 5;13:1203625. doi: 10.3389/fcimb.2023.1203625. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37736103 Free PMC article.
-
Rapid lateral flow tests for the detection of SARS-CoV-2 neutralizing antibodies.Expert Rev Mol Diagn. 2021 Apr;21(4):363-370. doi: 10.1080/14737159.2021.1913123. Epub 2021 Apr 12. Expert Rev Mol Diagn. 2021. PMID: 33840347 Free PMC article. Review.
-
SARS-CoV-2 spike S2-specific neutralizing antibodies.Emerg Microbes Infect. 2023 Dec;12(2):2220582. doi: 10.1080/22221751.2023.2220582. Emerg Microbes Infect. 2023. PMID: 37254830 Free PMC article. Review.
Cited by
-
Magnetic bead-based electrochemical surrogate virus neutralization test for quantification of antibody neutralizing efficiency.Biosens Bioelectron. 2025 Nov 1;287:117640. doi: 10.1016/j.bios.2025.117640. Epub 2025 May 30. Biosens Bioelectron. 2025. PMID: 40554384 Free PMC article.
-
Advances in Surrogate Neutralization Tests for High-Throughput Screening and the Point-of-Care.Anal Chem. 2025 Mar 18;97(10):5407-5423. doi: 10.1021/acs.analchem.5c00666. Epub 2025 Mar 4. Anal Chem. 2025. PMID: 40035475 Free PMC article. Review. No abstract available.
References
-
- Sarkar R.; Mitra S.; Chandra P.; Saha P.; Banerjee A.; Dutta S.; Chawla-Sarkar M. Comprehensive analysis of genomic diversity of SARS-CoV-2 in different geographic regions of India: an endeavour to classify Indian SARS-CoV-2 strains on the basis of co-existing mutations. Arch. Virol. 2021, 166 (3), 801–812. 10.1007/s00705-020-04911-0. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

