Leveraging the Stereochemical Complexity of Octahedral Diastereomeric-at-Metal Catalysts to Unlock Regio-, Diastereo-, and Enantioselectivity in Alcohol-Mediated C-C Couplings via Hydrogen Transfer
- PMID: 38478891
- PMCID: PMC11446212
- DOI: 10.1021/jacs.4c01857
Leveraging the Stereochemical Complexity of Octahedral Diastereomeric-at-Metal Catalysts to Unlock Regio-, Diastereo-, and Enantioselectivity in Alcohol-Mediated C-C Couplings via Hydrogen Transfer
Abstract
Experimental and computational studies illuminating the factors that guide metal-centered stereogenicity and, therefrom, selectivity in transfer hydrogenative carbonyl additions of alcohol proelectrophiles catalyzed by chiral-at-metal-and-ligand octahedral d6 metal ions, iridium(III) and ruthenium(II), are described. To augment or invert regio-, diastereo-, and enantioselectivity, predominantly one from among as many as 15 diastereomeric-at-metal complexes is required. For iridium(III) catalysts, cyclometalation assists in defining the metal stereocenter, and for ruthenium(II) catalysts, iodide counterions play a key role. Whereas classical strategies to promote selectivity in metal catalysis aim for high-symmetry transition states, well-defined low-symmetry transition states can unlock selectivities that are otherwise difficult to achieve or inaccessible.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

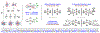

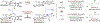
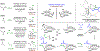


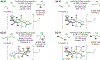
Similar articles
-
Understanding Halide Counterion Effects in Enantioselective Ruthenium-Catalyzed Carbonyl (α-Aryl)allylation: Alkynes as Latent Allenes and Trifluoroethanol-Enhanced Turnover in The Conversion of Ethanol to Higher Alcohols via Hydrogen Auto-transfer.J Am Chem Soc. 2021 Oct 13;143(40):16709-16717. doi: 10.1021/jacs.1c07857. Epub 2021 Oct 4. J Am Chem Soc. 2021. PMID: 34606271 Free PMC article.
-
Chiral-at-Ruthenium-SEGPHOS Catalysts Display Diastereomer-Dependent Regioselectivity: Enantioselective Isoprene-Mediated Carbonyl tert-Prenylation via Halide Counterion Effects.J Am Chem Soc. 2023 Aug 23;145(33):18676-18683. doi: 10.1021/jacs.3c06734. Epub 2023 Aug 9. J Am Chem Soc. 2023. PMID: 37555765 Free PMC article.
-
Cyclometalated Iridium-PhanePhos Complexes Are Active Catalysts in Enantioselective Allene-Fluoral Reductive Coupling and Related Alcohol-Mediated Carbonyl Additions That Form Acyclic Quaternary Carbon Stereocenters.J Am Chem Soc. 2019 Feb 6;141(5):2087-2096. doi: 10.1021/jacs.8b11868. Epub 2019 Jan 25. J Am Chem Soc. 2019. PMID: 30681850 Free PMC article.
-
Diastereo- and enantioselective anti-selective hydrogenation of α-amino-β-keto ester hydrochlorides and related compounds using transition-metal-chiral-bisphosphine catalysts.Chem Rec. 2014 Apr;14(2):235-50. doi: 10.1002/tcr.201300032. Epub 2014 Feb 18. Chem Rec. 2014. PMID: 24550034 Review.
-
Recent advances in osmium-catalyzed hydrogenation and dehydrogenation reactions.Acc Chem Res. 2015 Feb 17;48(2):363-79. doi: 10.1021/ar5003818. Epub 2015 Feb 4. Acc Chem Res. 2015. PMID: 25650714 Review.
Cited by
-
anti-Diastereo- and Enantioselective Ruthenium-Catalyzed C-C Coupling-Oxidative Lactonization of 1,4-Butanediol: Alkynes as Allylmetal Pronucleophiles.J Am Chem Soc. 2025 Aug 6;147(31):28370-28377. doi: 10.1021/jacs.5c09358. Epub 2025 Jul 28. J Am Chem Soc. 2025. PMID: 40719306 Free PMC article.
-
Dirhodium Complexes Heterochiral-at-the-Metal Centers: An Alternative Type of Paddlewheel Catalyst for Asymmetric Synthesis.J Am Chem Soc. 2025 Apr 16;147(15):12418-12424. doi: 10.1021/jacs.5c03567. Epub 2025 Apr 4. J Am Chem Soc. 2025. PMID: 40183523 Free PMC article.
-
Catalytic Enantioselective C-C Coupling of Alcohols for Polyketide Total Synthesis beyond Chiral Auxiliaries and Premetalated Reagents.Chem Rev. 2024 Dec 25;124(24):13715-13735. doi: 10.1021/acs.chemrev.4c00858. Epub 2024 Dec 6. Chem Rev. 2024. PMID: 39642170 Review.
-
β-Hydroxy Esters as Malonic Semialdehyde Proelectrophiles in Enantioselective Butadiene-Mediated Crotylation: Total Synthesis of Octalactins A and B.Org Lett. 2024 Jun 7;26(22):4830-4834. doi: 10.1021/acs.orglett.4c01644. Epub 2024 May 28. Org Lett. 2024. PMID: 38804715 Free PMC article.
-
Formal Synthesis of Fostriecin via Asymmetric Alcohol-Mediated Carbonyl Allylation.Org Lett. 2025 May 2;27(17):4501-4506. doi: 10.1021/acs.orglett.5c01026. Epub 2025 Apr 10. Org Lett. 2025. PMID: 40209063 Free PMC article.
References
-
- For reviews, see: (a) Trost BM The Atom Economy—A Search for Synthetic Efficiency. Science 1991, 254, 1471–1477. - PubMed
- (b) Doerksen RS; Meyer CC; Krische MJ Feedstock Reagents in Metal-Catalyzed Carbonyl Reductive Coupling: Minimizing Preactivation for Efficiency in Target-Oriented Synthesis. Angew. Chem. Int. Ed 2019, 58, 14055–14064 and references cited therein. - PMC - PubMed
-
- For selected reviews on enantioselective hydrogenation and transfer hydrogenation in the synthesis of pharmaceutical ingredients, see: (a) Hawkins JM; Watson TJN Asymmetric Catalysis in the Pharmaceutical Industry. Angew. Chem. Int. Ed 2004, 43, 3224–3228. - PubMed
- (b) Thommen M Homogeneous Asymmetric Hydrogenation: Mature and Fit for Early Stage Drug Development. Spec. Chem. Mag 2005, 25, 26–28.
- (c) Thayer AM Chiral Catalysis. Chem. Eng. News 2005, 83, 40–58.
- (d) Farina V; Reeves JT; Senanayake CH; Song JJ Asymmetric Synthesis of Active Pharmaceutical Ingredients. Chem. Rev 2006, 106, 2734–2793. - PubMed
- (e) Carey JS; Laffan D; Thomson C; Williams MT Analysis of The Reactions Used for The Preparation of Drug Candidate Molecules. Org. Biomol. Chem 2006, 4, 2337–2347. - PubMed
- (f) Ager DJ; de Vries AHM; de Vries JG Asymmetric Homogeneous Hydrogenations at Scale. Chem. Soc. Rev 2012, 41, 3340–3380. - PubMed
- (g) Etayo P; Vidal-Ferran A Rhodium-Catalyzed Asymmetric Hydrogenation as a Valuable Synthetic Tool for The Preparation of Chiral Drugs. Chem. Soc. Rev 2013, 42, 728–754. - PubMed
- (h) Hayler JD; Leahy DK; Simmons EM A Pharmaceutical Industry Perspective on Sustainable Metal Catalysis. Organometallics 2019, 38, 36–46.
-
- For a review on the Haber-Bosch process, see: Smil V Enriching the Earth: Fritz Haber, Carl Bosch, and the Transformation of World Food Production, MIT Press: Cambridge, MA, 2004; pp 68–107.
-
- For a review on methane-steam reforming, see: Hook van J. P. Methane-Steam Reforming. Catal. Rev 1980, 21, 1–51.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

