Differential clustering of visual and choice- and saccade-related activity in macaque V3A and CIP
- PMID: 38478896
- PMCID: PMC11305645
- DOI: 10.1152/jn.00285.2023
Differential clustering of visual and choice- and saccade-related activity in macaque V3A and CIP
Abstract
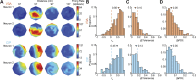
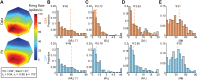
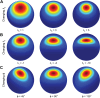
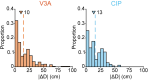
Neurons in sensory and motor cortices tend to aggregate in clusters with similar functional properties. Within the primate dorsal ("where") pathway, an important interface between three-dimensional (3-D) visual processing and motor-related functions consists of two hierarchically organized areas: V3A and the caudal intraparietal (CIP) area. In these areas, 3-D visual information, choice-related activity, and saccade-related activity converge, often at the single-neuron level. Characterizing the clustering of functional properties in areas with mixed selectivity, such as these, may help reveal organizational principles that support sensorimotor transformations. Here we quantified the clustering of visual feature selectivity, choice-related activity, and saccade-related activity by performing correlational and parametric comparisons of the responses of well-isolated, simultaneously recorded neurons in macaque monkeys. Each functional domain showed statistically significant clustering in both areas. However, there were also domain-specific differences in the strength of clustering across the areas. Visual feature selectivity and saccade-related activity were more strongly clustered in V3A than in CIP. In contrast, choice-related activity was more strongly clustered in CIP than in V3A. These differences in clustering may reflect the areas' roles in sensorimotor processing. Stronger clustering of visual and saccade-related activity in V3A may reflect a greater role in within-domain processing, as opposed to cross-domain synthesis. In contrast, stronger clustering of choice-related activity in CIP may reflect a greater role in synthesizing information across functional domains to bridge perception and action.NEW & NOTEWORTHY The occipital and parietal cortices of macaque monkeys are bridged by hierarchically organized areas V3A and CIP. These areas support 3-D visual transformations, carry choice-related activity during 3-D perceptual tasks, and possess saccade-related activity. This study quantifies the functional clustering of neuronal response properties within V3A and CIP for each of these domains. The findings reveal domain-specific cross-area differences in clustering that may reflect the areas' roles in sensorimotor processing.
Keywords: choice signals; neuronal clustering; oculomotor; sensorimotor; vision.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures




Similar articles
-
Parallel processing, hierarchical transformations, and sensorimotor associations along the 'where' pathway.Elife. 2022 Aug 11;11:e78712. doi: 10.7554/eLife.78712. Elife. 2022. PMID: 35950921 Free PMC article.
-
Choice-Related Activity during Visual Slant Discrimination in Macaque CIP But Not V3A.eNeuro. 2019 Mar 26;6(2):ENEURO.0248-18.2019. doi: 10.1523/ENEURO.0248-18.2019. eCollection 2019 Mar-Apr. eNeuro. 2019. PMID: 30923736 Free PMC article.
-
Visual, saccade-related, and cognitive activation of single neurons in monkey extrastriate area V3A.J Neurophysiol. 2000 Aug;84(2):677-92. doi: 10.1152/jn.2000.84.2.677. J Neurophysiol. 2000. PMID: 10938295
-
Linking express saccade occurance to stimulus properties and sensorimotor integration in the superior colliculus.J Neurophysiol. 2015 Aug;114(2):879-92. doi: 10.1152/jn.00047.2015. Epub 2015 Jun 10. J Neurophysiol. 2015. PMID: 26063770 Free PMC article.
-
Updating of the visual representation in monkey striate and extrastriate cortex during saccades.Proc Natl Acad Sci U S A. 2002 Mar 19;99(6):4026-31. doi: 10.1073/pnas.052379899. Proc Natl Acad Sci U S A. 2002. PMID: 11904446 Free PMC article.
Cited by
-
Efferent compared to afferent neural substrates of the vergence eye movement system evoked via fMRI.Front Neurosci. 2025 Jan 8;18:1497326. doi: 10.3389/fnins.2024.1497326. eCollection 2024. Front Neurosci. 2025. PMID: 39844855 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

