De novo variants in FRYL are associated with developmental delay, intellectual disability, and dysmorphic features
- PMID: 38479391
- PMCID: PMC11023917
- DOI: 10.1016/j.ajhg.2024.02.007
De novo variants in FRYL are associated with developmental delay, intellectual disability, and dysmorphic features
Abstract
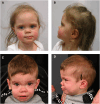
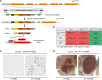
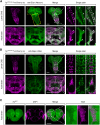
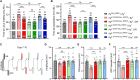
FRY-like transcription coactivator (FRYL) belongs to a Furry protein family that is evolutionarily conserved from yeast to humans. The functions of FRYL in mammals are largely unknown, and variants in FRYL have not previously been associated with a Mendelian disease. Here, we report fourteen individuals with heterozygous variants in FRYL who present with developmental delay, intellectual disability, dysmorphic features, and other congenital anomalies in multiple systems. The variants are confirmed de novo in all individuals except one. Human genetic data suggest that FRYL is intolerant to loss of function (LoF). We find that the fly FRYL ortholog, furry (fry), is expressed in multiple tissues, including the central nervous system where it is present in neurons but not in glia. Homozygous fry LoF mutation is lethal at various developmental stages, and loss of fry in mutant clones causes defects in wings and compound eyes. We next modeled four out of the five missense variants found in affected individuals using fry knockin alleles. One variant behaves as a severe LoF variant, whereas two others behave as partial LoF variants. One variant does not cause any observable defect in flies, and the corresponding human variant is not confirmed to be de novo, suggesting that this is a variant of uncertain significance. In summary, our findings support that fry is required for proper development in flies and that the LoF variants in FRYL cause a dominant disorder with developmental and neurological symptoms due to haploinsufficiency.
Keywords: Drosophila; FRYL; developmental delay; furry; intellectual disability; rare disease.
Copyright © 2024 American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests W.K.C. is a member of the Board of Directors of Prime Medicine and RallyBio. R.P. is an employee of GeneDx, LLC.
Figures


References
-
- Hirata D., Kishimoto N., Suda M., Sogabe Y., Nakagawa S., Yoshida Y., Sakai K., Mizunuma M., Miyakawa T., Ishiguro J., Toda T. Fission yeast Mor2/Cps12, a protein similar to Drosophila Furry, is essential for cell morphogenesis and its mutation induces Wee1-dependent G(2) delay. EMBO J. 2002;21:4863–4874. doi: 10.1093/emboj/cdf495. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

