Pathophysiology of chikungunya virus infection associated with fatal outcomes
- PMID: 38479396
- PMCID: PMC11018361
- DOI: 10.1016/j.chom.2024.02.011
Pathophysiology of chikungunya virus infection associated with fatal outcomes
Abstract
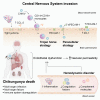
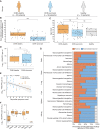
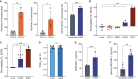
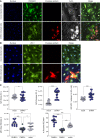
Chikungunya virus (CHIKV) is a mosquito-borne alphavirus that causes acute, subacute, and chronic human arthritogenic diseases and, in rare instances, can lead to neurological complications and death. Here, we combined epidemiological, virological, histopathological, cytokine, molecular dynamics, metabolomic, proteomic, and genomic analyses to investigate viral and host factors that contribute to chikungunya-associated (CHIK) death. Our results indicate that CHIK deaths are associated with multi-organ infection, central nervous system damage, and elevated serum levels of pro-inflammatory cytokines and chemokines compared with survivors. The histopathologic, metabolite, and proteomic signatures of CHIK deaths reveal hemodynamic disorders and dysregulated immune responses. The CHIKV East-Central-South-African lineage infecting our study population causes both fatal and survival cases. Additionally, CHIKV infection impairs the integrity of the blood-brain barrier, as evidenced by an increase in permeability and altered tight junction protein expression. Overall, our findings improve the understanding of CHIK pathophysiology and the causes of fatal infections.
Keywords: alphavirus; arbovirus; central nervous system infection; chikungunya death; chikungunya virus; hemodynamic disorders; inflammation; mosquito-borne virus; pathophysiology.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests M.S.D. is a consultant or advisor for Inbios, Ocugen, Vir Biotechnology, Topspin Therapeutics, Moderna, Merck, and Immunome. The Diamond laboratory has received funding support from Emergent BioSolutions, Moderna, and Vir Biotechnology.
Figures




References
-
- Suhrbier A. Rheumatic manifestations of chikungunya: emerging concepts and interventions. Nat. Rev. Rheumatol. 2019;15:597–611. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

