Acidity of persulfides and its modulation by the protein environments in sulfide quinone oxidoreductase and thiosulfate sulfurtransferase
- PMID: 38479599
- PMCID: PMC11039317
- DOI: 10.1016/j.jbc.2024.107149
Acidity of persulfides and its modulation by the protein environments in sulfide quinone oxidoreductase and thiosulfate sulfurtransferase
Abstract
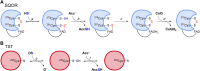
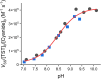
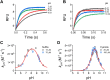
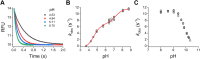
Persulfides (RSSH/RSS-) participate in sulfur metabolism and are proposed to transduce hydrogen sulfide (H2S) signaling. Their biochemical properties are poorly understood. Herein, we studied the acidity and nucleophilicity of several low molecular weight persulfides using the alkylating agent, monobromobimane. The different persulfides presented similar pKa values (4.6-6.3) and pH-independent rate constants (3.2-9.0 × 103 M-1 s-1), indicating that the substituents in persulfides affect properties to a lesser extent than in thiols because of the larger distance to the outer sulfur. The persulfides had higher reactivity with monobromobimane than analogous thiols and putative thiols with the same pKa, providing evidence for the alpha effect (enhanced nucleophilicity by the presence of a contiguous atom with high electron density). Additionally, we investigated two enzymes from the human mitochondrial H2S oxidation pathway that form catalytic persulfide intermediates, sulfide quinone oxidoreductase and thiosulfate sulfurtransferase (TST, rhodanese). The pH dependence of the activities of both enzymes was measured using sulfite and/or cyanide as sulfur acceptors. The TST half-reactions were also studied by stopped-flow fluorescence spectroscopy. Both persulfidated enzymes relied on protonated groups for reaction with the acceptors. Persulfidated sulfide quinone oxidoreductase appeared to have a pKa of 7.8 ± 0.2. Persulfidated TST presented a pKa of 9.38 ± 0.04, probably due to a critical active site residue rather than the persulfide itself. The TST thiol reacted in the anionic state with thiosulfate, with an apparent pKa of 6.5 ± 0.1. Overall, our study contributes to a fundamental understanding of persulfide properties and their modulation by protein environments.
Keywords: alpha effect; hydrogen sulfide; pK(a); persulfide; rhodanese; sulfide quinone oxidoreductase; thiol; thiosulfate sulfurtransferase.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures




References
-
- Wood J.L. Sulfane sulfur. Methods Enzymol. 1987;143:25–29. - PubMed
-
- Bouillaud F., Blachier F. Mitochondria and sulfide: a very old story of poisoning, feeding, and signaling? Antioxid. Redox Signal. 2011;15:379–391. - PubMed
-
- Mueller E.G. Trafficking in persulfides: delivering sulfur in biosynthetic pathways. Nat. Chem. Biol. 2006;2:185–194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

