The aryl hydrocarbon receptor-interacting protein in cancer and immunity: Beyond a chaperone protein for the dioxin receptor
- PMID: 38479600
- PMCID: PMC11002312
- DOI: 10.1016/j.jbc.2024.107157
The aryl hydrocarbon receptor-interacting protein in cancer and immunity: Beyond a chaperone protein for the dioxin receptor
Abstract
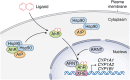
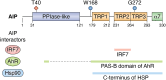
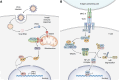
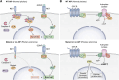
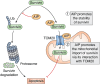
The aryl hydrocarbon receptor (AhR)-interacting protein (AIP) is a ubiquitously expressed, immunophilin-like protein best known for its role as a co-chaperone in the AhR-AIP-Hsp90 cytoplasmic complex. In addition to regulating AhR and the xenobiotic response, AIP has been linked to various aspects of cancer and immunity that will be the focus of this review article. Loss-of-function AIP mutations are associated with pituitary adenomas, suggesting that AIP acts as a tumor suppressor in the pituitary gland. However, the tumor suppressor mechanisms of AIP remain unclear, and AIP can exert oncogenic functions in other tissues. While global deletion of AIP in mice yields embryonically lethal cardiac malformations, heterozygote, and tissue-specific conditional AIP knockout mice have revealed various physiological roles of AIP. Emerging studies have established the regulatory roles of AIP in both innate and adaptive immunity. AIP interacts with and inhibits the nuclear translocation of the transcription factor IRF7 to inhibit type I interferon production. AIP also interacts with the CARMA1-BCL10-MALT1 complex in T cells to enhance IKK/NF-κB signaling and T cell activation. Taken together, AIP has diverse functions that vary considerably depending on the client protein, the tissue, and the species.
Keywords: aryl hydrocarbon receptor (AhR); aryl hydrocarbon receptor-interacting protein (AIP); cancer; chaperones; immunity; pituitary adenoma.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
The aryl hydrocarbon receptor-interacting protein (AIP) is required for dioxin-induced hepatotoxicity but not for the induction of the Cyp1a1 and Cyp1a2 genes.J Biol Chem. 2010 Nov 12;285(46):35599-605. doi: 10.1074/jbc.M110.132043. Epub 2010 Sep 9. J Biol Chem. 2010. PMID: 20829355 Free PMC article.
-
Expression of aryl hydrocarbon receptor (AHR) and AHR-interacting protein in pituitary adenomas: pathological and clinical implications.Endocr Relat Cancer. 2009 Sep;16(3):1029-43. doi: 10.1677/ERC-09-0094. Epub 2009 Jun 25. Endocr Relat Cancer. 2009. PMID: 19556287
-
AIP mutations impair AhR signaling in pituitary adenoma patients fibroblasts and in GH3 cells.Endocr Relat Cancer. 2016 May;23(5):433-43. doi: 10.1530/ERC-16-0041. Epub 2016 Apr 14. Endocr Relat Cancer. 2016. PMID: 27080473
-
AIP and its interacting partners.J Endocrinol. 2011 Aug;210(2):137-55. doi: 10.1530/JOE-11-0054. Epub 2011 Mar 31. J Endocrinol. 2011. PMID: 21454441 Review.
-
Familial isolated pituitary adenomas (FIPA) and the pituitary adenoma predisposition due to mutations in the aryl hydrocarbon receptor interacting protein (AIP) gene.Endocr Rev. 2013 Apr;34(2):239-77. doi: 10.1210/er.2012-1013. Epub 2013 Jan 31. Endocr Rev. 2013. PMID: 23371967 Free PMC article. Review.
Cited by
-
Ultra-Processed Diets and Endocrine Disruption, Explanation of Missing Link in Rising Cancer Incidence Among Young Adults.Cancers (Basel). 2025 Jun 29;17(13):2196. doi: 10.3390/cancers17132196. Cancers (Basel). 2025. PMID: 40647494 Free PMC article. Review.
-
MALT1 Inhibitors for Treating Cancer and Immunological Diseases.ACS Med Chem Lett. 2024 Aug 20;15(9):1419-1420. doi: 10.1021/acsmedchemlett.4c00390. eCollection 2024 Sep 12. ACS Med Chem Lett. 2024. PMID: 39291001
-
The aryl hydrocarbon receptor interacting protein suppresses RNA virus-induced type I IFN and IL-6 in mouse macrophages.bioRxiv [Preprint]. 2025 Jul 2:2025.06.30.662398. doi: 10.1101/2025.06.30.662398. bioRxiv. 2025. PMID: 40631146 Free PMC article. Preprint.
-
Targeted protein degradation with small molecules for cancer immunotherapy.Asian J Pharm Sci. 2025 Aug;20(4):101058. doi: 10.1016/j.ajps.2025.101058. Epub 2025 Apr 22. Asian J Pharm Sci. 2025. PMID: 40791660 Free PMC article. Review.
References
-
- Meyer B.K., Perdew G.H. Characterization of the AhR-hsp90-XAP2 core complex and the role of the immunophilin-related protein XAP2 in AhR stabilization. Biochemistry. 1999;38:8907–8917. - PubMed
-
- Ma Q., Whitlock J.P., Jr. A novel cytoplasmic protein that interacts with the Ah receptor, contains tetratricopeptide repeat motifs, and augments the transcriptional response to 2,3,7,8-tetrachlorodibenzo-p-dioxin. J. Biol. Chem. 1997;272:8878–8884. - PubMed
-
- Lees M.J., Peet D.J., Whitelaw M.L. Defining the role for XAP2 in stabilization of the dioxin receptor. J. Biol. Chem. 2003;278:35878–35888. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

