Understanding and maximising the community impact of seasonal malaria chemoprevention in Burkina Faso (INDIE-SMC): study protocol for a cluster randomised evaluation trial
- PMID: 38479748
- PMCID: PMC10936478
- DOI: 10.1136/bmjopen-2023-081682
Understanding and maximising the community impact of seasonal malaria chemoprevention in Burkina Faso (INDIE-SMC): study protocol for a cluster randomised evaluation trial
Abstract
Introduction: Seasonal malaria chemoprevention (SMC) involves repeated administrations of sulfadoxine-pyrimethamine plus amodiaquine to children below the age of 5 years during the peak transmission season in areas of seasonal malaria transmission. While highly impactful in reducing Plasmodium falciparum malaria burden in controlled research settings, the impact of SMC on infection prevalence is moderate in real-life settings. It remains unclear what drives this efficacy decay. Recently, the WHO widened the scope for SMC to target all vulnerable populations. The Ministry of Health (MoH) in Burkina Faso is considering extending SMC to children below 10 years old. We aim to assess the impact of SMC on clinical incidence and parasite prevalence and quantify the human infectious reservoir for malaria in this population.
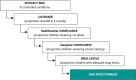
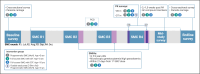
Methods and analysis: We will perform a cluster randomised trial in Saponé Health District, Burkina Faso, with three study arms comprising 62 clusters of three compounds: arm 1 (control): SMC in under 5-year-old children, implemented by the MoH without directly observed treatment (DOT) for the full course of SMC; arm 2 (intervention): SMC in under 5-year-old children, with DOT for the full course of SMC; arm 3 (intervention): SMC in under 10-year-old children, with DOT for the full course of SMC. The primary endpoint is parasite prevalence at the end of the malaria transmission season. Secondary endpoints include the impact of SMC on clinical incidence. Factors affecting SMC uptake, treatment adherence, drug concentrations, parasite resistance markers and transmission of parasites will be determined.
Ethics and dissemination: The London School of Hygiene & Tropical Medicine's Ethics Committee (29193) and the Burkina Faso National Medical Ethics Committee (Deliberation No 2023-05-104) approved this study. The findings will be presented to the community; disease occurrence data and study outcomes will also be shared with the Burkina Faso MoH. Findings will be published irrespective of their results.
Trial registration number: NCT05878366.
Keywords: EPIDEMIOLOGIC STUDIES; Malaria; Tropical medicine.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
-
- Organization WH . World malaria report 2022. Contract no.: CC BY-NC-SA 3.0 IGO; 2022.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
