Brain sodium sensing for regulation of thirst, salt appetite, and blood pressure
- PMID: 38479999
- PMCID: PMC10937250
- DOI: 10.14814/phy2.15970
Brain sodium sensing for regulation of thirst, salt appetite, and blood pressure
Abstract
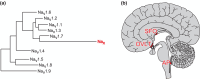
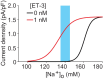
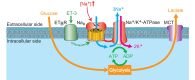
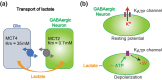
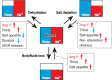
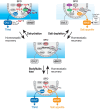
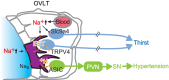
The brain possesses intricate mechanisms for monitoring sodium (Na) levels in body fluids. During prolonged dehydration, the brain detects variations in body fluids and produces sensations of thirst and aversions to salty tastes. At the core of these processes Nax , the brain's Na sensor, exists. Specialized neural nuclei, namely the subfornical organ (SFO) and organum vasculosum of the lamina terminalis (OVLT), which lack the blood-brain barrier, play pivotal roles. Within the glia enveloping the neurons in these regions, Nax collaborates with Na+ /K+ -ATPase and glycolytic enzymes to drive glycolysis in response to elevated Na levels. Lactate released from these glia cells activates nearby inhibitory neurons. The SFO hosts distinct types of angiotensin II-sensitive neurons encoding thirst and salt appetite, respectively. During dehydration, Nax -activated inhibitory neurons suppress salt-appetite neuron's activity, whereas salt deficiency reduces thirst neuron's activity through cholecystokinin. Prolonged dehydration increases the Na sensitivity of Nax via increased endothelin expression in the SFO. So far, patients with essential hypernatremia have been reported to lose thirst and antidiuretic hormone release due to Nax -targeting autoantibodies. Inflammation in the SFO underlies the symptoms. Furthermore, Nax activation in the OVLT, driven by Na retention, stimulates the sympathetic nervous system via acid-sensing ion channels, contributing to a blood pressure elevation.
Keywords: Nax; OVLT; blood pressure; salt appetite; subfornical organ; thirst.
© 2024 The Authors. Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Integration of Hypernatremia and Angiotensin II by the Organum Vasculosum of the Lamina Terminalis Regulates Thirst.J Neurosci. 2020 Mar 4;40(10):2069-2079. doi: 10.1523/JNEUROSCI.2208-19.2020. Epub 2020 Jan 31. J Neurosci. 2020. PMID: 32005766 Free PMC article.
-
Distinct neural mechanisms for the control of thirst and salt appetite in the subfornical organ.Nat Neurosci. 2017 Feb;20(2):230-241. doi: 10.1038/nn.4463. Epub 2016 Dec 19. Nat Neurosci. 2017. PMID: 27991901
-
Sodium sensing in the brain.Pflugers Arch. 2015 Mar;467(3):465-74. doi: 10.1007/s00424-014-1662-4. Epub 2014 Dec 10. Pflugers Arch. 2015. PMID: 25491503 Free PMC article. Review.
-
The organum vasculosum of the lamina terminalis and subfornical organ: regulation of thirst.Front Neurosci. 2023 Sep 4;17:1223836. doi: 10.3389/fnins.2023.1223836. eCollection 2023. Front Neurosci. 2023. PMID: 37732311 Free PMC article. Review.
-
SLC9A4 in the organum vasculosum of the lamina terminalis is a [Na+] sensor for the control of water intake.Pflugers Arch. 2020 May;472(5):609-624. doi: 10.1007/s00424-020-02389-y. Epub 2020 May 6. Pflugers Arch. 2020. PMID: 32372285
Cited by
-
The relationship between dehydration and etiologic subtypes of major neurocognitive disorder in older patients.Eur Geriatr Med. 2024 Aug;15(4):1159-1168. doi: 10.1007/s41999-024-00986-z. Epub 2024 May 17. Eur Geriatr Med. 2024. PMID: 38755401
-
Central nervous system mechanisms of salt-sensitive hypertension.Physiol Rev. 2025 Oct 1;105(4):1989-2032. doi: 10.1152/physrev.00035.2024. Epub 2025 May 2. Physiol Rev. 2025. PMID: 40315132 Review.
References
-
- Broadwell, R. D. , & Sofroniew, M. V. (1993). Serum proteins bypass the blood‐brain fluid barriers for extracellular entry to the central nervous system. Experimental Neurology, 120, 245–263. - PubMed
-
- Buggy, J. , & Fisher, A. E. (1974). Evidence for a dual central role for angiotensin in water and sodium intake. Nature, 250, 733–735. - PubMed
-
- Fitzsimons, J. T. (1998). Angiotensin, thirst, and sodium appetite. Physiological Reviews, 78, 583–686. - PubMed
-
- Gautron, S. , Dos Santos, G. , Pinto‐Henrique, D. , Koulakoff, A. , Gros, F. , & Berwald‐Netter, Y. (1992). The glial voltage‐gated sodium channel: Cell‐ and tissue‐specific mRNA expression. Proceedings of the National Academy of Sciences of the United States of America, 89, 7272–7276. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

