Upregulation of HLA-II related to LAG-3+CD4+ T cell infiltration is associated with patient outcome in human glioblastoma
- PMID: 38480275
- PMCID: PMC11093187
- DOI: 10.1111/cas.16128
Upregulation of HLA-II related to LAG-3+CD4+ T cell infiltration is associated with patient outcome in human glioblastoma
Erratum in
-
Correction to "Upregulation of HLA-II Related to LAG-3+CD4+ T Cell Infiltration is Associated With Patient Outcome in Human Glioblastoma".Cancer Sci. 2025 Jul 1. doi: 10.1111/cas.70135. Online ahead of print. Cancer Sci. 2025. PMID: 40598684 No abstract available.
Abstract
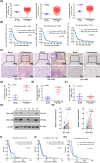
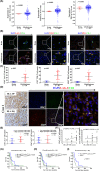
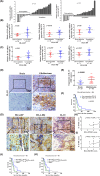
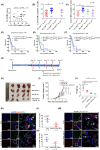
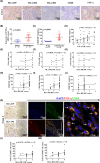
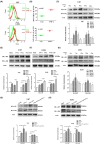
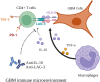
Glioblastoma (GBM) is the most common malignant diffuse glioma of the brain. Although immunotherapy with immune checkpoint inhibitors (ICIs), such as programmed cell death protein (PD)-1/PD ligand-1 inhibitors, has revolutionized the treatment of several cancers, the clinical benefit in GBM patients has been limited. Lymphocyte-activation gene 3 (LAG-3) binding to human leukocyte antigen-II (HLA-II) plays an essential role in triggering CD4+ T cell exhaustion and could interfere with the efficiency of anti-PD-1 treatment; however, the value of LAG-3-HLA-II interactions in ICI immunotherapy for GBM patients has not yet been analyzed. Therefore, we aimed to investigate the expression and regulation of HLA-II in human GBM samples and the correlation with LAG-3+CD4+ T cell infiltration. Human leukocyte antigen-II was highly expressed in GBM and correlated with increased LAG-3+CD4+ T cell infiltration in the stroma. Additionally, HLA-IIHighLAG-3High was associated with worse patient survival. Increased interleukin-10 (IL-10) expression was observed in GBM, which was correlated with high levels of HLA-II and LAG-3+ T cell infiltration in stroma. HLA-IIHighIL-10High GBM associated with LAG-3+ T cells infiltration synergistically showed shorter overall survival in patients. Combined anti-LAG-3 and anti-IL-10 treatment inhibited tumor growth in a mouse brain GL261 tumor model. In vitro, CD68+ macrophages upregulated HLA-II expression in GBM cells through tumor necrosis factor-α (TNF-α). Blocking TNF-α-dependent inflammation inhibited tumor growth in a mouse GBM model. In summary, T cell-tumor cell interactions, such as LAG-3-HLA-II, could confer an immunosuppressive environment in human GBM, leading to poor prognosis in patients. Therefore, targeting the LAG-3-HLA-II interaction could be beneficial in ICI immunotherapy to improve the clinical outcome of GBM patients.
Keywords: HLA‐II; IL‐10; LAG‐3; glioblastoma; immune checkpoint inhibitor.
© 2024 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Autophagy-related CMTM6 promotes glioblastoma progression by activating Wnt/β-catenin pathway and acts as an onco-immunological biomarker.J Gene Med. 2024 May;26(5):e3685. doi: 10.1002/jgm.3685. J Gene Med. 2024. PMID: 38686653
-
Single-dose radiotherapy is more effective than fractionation when combined with anti-PD-1 immunotherapy in glioblastoma.Sci Rep. 2025 Jul 2;15(1):22910. doi: 10.1038/s41598-025-06909-7. Sci Rep. 2025. PMID: 40596223 Free PMC article.
-
TNFSF14 and CD44 are overexpressed in glioblastoma and associated with immunosuppressive microenvironment.Biomol Biomed. 2025 Jul 31;25(8):1829-1843. doi: 10.17305/bb.2025.11791. Biomol Biomed. 2025. PMID: 39977830
-
Immune checkpoint inhibitors for glioblastoma: emerging science, clinical advances, and future directions.J Neurooncol. 2025 Feb;171(3):531-547. doi: 10.1007/s11060-024-04881-2. Epub 2024 Nov 21. J Neurooncol. 2025. PMID: 39570554 Free PMC article. Review.
-
Immune infiltration of tumor microenvironment following immunotherapy for glioblastoma multiforme.Hum Vaccin Immunother. 2017 Nov 2;13(11):2575-2582. doi: 10.1080/21645515.2017.1303582. Epub 2017 Mar 31. Hum Vaccin Immunother. 2017. PMID: 28362548 Free PMC article.
Cited by
-
Overcoming immunotherapy resistance in glioblastoma: challenges and emerging strategies.Front Pharmacol. 2025 Mar 28;16:1584688. doi: 10.3389/fphar.2025.1584688. eCollection 2025. Front Pharmacol. 2025. PMID: 40223940 Free PMC article. Review.
-
Barriers to T Cell Functionality in the Glioblastoma Microenvironment.Cancers (Basel). 2024 Sep 26;16(19):3273. doi: 10.3390/cancers16193273. Cancers (Basel). 2024. PMID: 39409893 Free PMC article. Review.
References
-
- Tan AC, Ashley DM, López GY, Malinzak M, Friedman HS, Khasraw M. Management of glioblastoma: state of the art and future directions[J]. CA Cancer J Clin. 2020;70(4):299‐312. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

