Alix-normalized exosomal programmed death-ligand 1 analysis in urine enables precision monitoring of urothelial cancer
- PMID: 38480462
- PMCID: PMC11093207
- DOI: 10.1111/cas.16106
Alix-normalized exosomal programmed death-ligand 1 analysis in urine enables precision monitoring of urothelial cancer
Abstract
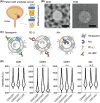
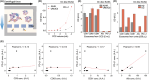
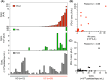
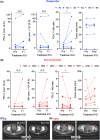
Anti-programmed death-ligand 1 (PD-L1) Ab-based therapies have demonstrated potential for treating metastatic urothelial cancer with high PD-L1 expression. Urinary exosomes are promising biomarkers for liquid biopsy, but urine's high variability requires normalization for accurate analysis. This study proposes using the PD-L1/Alix ratio to normalize exosomal PD-L1 signal intensity with Alix, an internal exosomal protein less susceptible to heterogeneity concerns than surface protein markers. Extracellular vesicles were isolated using ExoDisc and characterized using various methods, including ExoView to analyze tetraspanins, PD-L1, and Alix on individual exosomes. On-disc ELISA was used to evaluate PD-L1 and Alix-normalized PD-L1 in 15 urothelial cancer patients during the initial treatment cycle with Tecentriq. Results showed that Alix signal range was relatively uniform, whereas tetraspanin marker intensity varied for individual exosome particles. On-disc ELISA was more reliable for detecting exosomal PD-L1 expression than standard plate ELISA-based measurement. Using exosomal Alix expression for normalization is a more reliable approach than conventional methods for monitoring patient status. Overall, the study provides a practical and reliable method for detecting exosomal PD-L1 in urine samples from patients with urothelial cancer.
Keywords: PD‐L1; exosome; normalization; urine; urothelial cancer.
© 2024 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
UNIST has filed patents on ExoDisc, and Yoon‐Kyoung Cho is named as an inventor, which are licensed to LabSpinner, Inc. Yoon‐Kyoung Cho is a stockholder of LabSpinner, Inc. No potential conflicts of interest were disclosed by the other authors.
Figures
Similar articles
-
ALIX Regulates Tumor-Mediated Immunosuppression by Controlling EGFR Activity and PD-L1 Presentation.Cell Rep. 2018 Jul 17;24(3):630-641. doi: 10.1016/j.celrep.2018.06.066. Cell Rep. 2018. PMID: 30021161 Free PMC article.
-
Exosomal PD-L1 and N-cadherin predict pulmonary metastasis progression for osteosarcoma patients.J Nanobiotechnology. 2020 Oct 22;18(1):151. doi: 10.1186/s12951-020-00710-6. J Nanobiotechnology. 2020. PMID: 33092576 Free PMC article.
-
Prostate cancer cells synergistically defend against CD8+ T cells by secreting exosomal PD-L1.Cancer Med. 2023 Aug;12(15):16405-16415. doi: 10.1002/cam4.6275. Epub 2023 Jul 27. Cancer Med. 2023. PMID: 37501397 Free PMC article.
-
The Importance of Exosomal PD-L1 in Cancer Progression and Its Potential as a Therapeutic Target.Cells. 2021 Nov 19;10(11):3247. doi: 10.3390/cells10113247. Cells. 2021. PMID: 34831468 Free PMC article. Review.
-
Clinical Implications of Exosomal PD-L1 in Cancer Immunotherapy.J Immunol Res. 2021 Feb 8;2021:8839978. doi: 10.1155/2021/8839978. eCollection 2021. J Immunol Res. 2021. PMID: 33628854 Free PMC article. Review.
Cited by
-
Enhancing outcome prediction of concurrent chemoradiation treatment in patients with locally advanced cervical cancer through plasma extracellular vesicle proteomics.Heliyon. 2024 Aug 22;10(16):e36374. doi: 10.1016/j.heliyon.2024.e36374. eCollection 2024 Aug 30. Heliyon. 2024. PMID: 39262965 Free PMC article.
-
Exosomal PD-L1 in cancer and other fields: recent advances and perspectives.Front Immunol. 2024 Apr 25;15:1395332. doi: 10.3389/fimmu.2024.1395332. eCollection 2024. Front Immunol. 2024. PMID: 38726017 Free PMC article. Review.
-
Label-free Detection of Urine Extracellular Vesicles from Duchenne Muscular Dystrophy Patients Using Surface-Enhanced Raman Spectroscopy Combined with Machine Learning Models.ACS Omega. 2025 Mar 24;10(16):16874-16883. doi: 10.1021/acsomega.5c00838. eCollection 2025 Apr 29. ACS Omega. 2025. PMID: 40321535 Free PMC article.
-
Recent Exploration of Solid Cancer Biomarkers Hidden Within Urine or Blood Exosomes That Provide Fundamental Information for Future Cancer Diagnostics.Diagnostics (Basel). 2025 Mar 5;15(5):628. doi: 10.3390/diagnostics15050628. Diagnostics (Basel). 2025. PMID: 40075875 Free PMC article. Review.
References
-
- Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti‐PD‐L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558‐562. - PubMed
-
- Baietti MF, Zhang Z, Mortier E, et al. Syndecan‐syntenin‐ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14(7):677‐685. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

