Enantioselective Sulfonimidamide Acylation via a Cinchona Alkaloid-Catalyzed Desymmetrization: Scope, Data Science, and Mechanistic Investigation
- PMID: 38480482
- PMCID: PMC10990064
- DOI: 10.1021/jacs.4c00374
Enantioselective Sulfonimidamide Acylation via a Cinchona Alkaloid-Catalyzed Desymmetrization: Scope, Data Science, and Mechanistic Investigation
Abstract
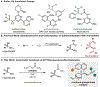
Methods to access chiral sulfur(VI) pharmacophores are of interest in medicinal and synthetic chemistry. We report the desymmetrization of unprotected sulfonimidamides via asymmetric acylation with a cinchona-phosphinate catalyst. The desired products are formed in excellent yield and enantioselectivity with no observed bis-acylation. A data-science-driven approach to substrate scope evaluation was coupled to high throughput experimentation (HTE) to facilitate statistical modeling in order to inform mechanistic studies. Reaction kinetics, catalyst structural studies, and density functional theory (DFT) transition state analysis elucidated the turnover-limiting step to be the collapse of the tetrahedral intermediate and provided key insights into the catalyst-substrate structure-activity relationships responsible for the origin of the enantioselectivity. This study offers a reliable method for accessing enantioenriched sulfonimidamides to propel their application as pharmacophores and serves as an example of the mechanistic insight that can be gleaned from integrating data science and traditional physical organic techniques.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






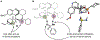


References
-
- Tilby MJ; Willis MC How do we address neglected sulfur pharmacophores in drug discovery? Expert Opin. Drug Discovery 2021, 16, 1227–1231. - PubMed
-
- Foote KM; Nissink JWM; McGuire T; Turner P; Guichard S; Yates JWT; Lau A; Blades K; Heathcote D; Odedra R; Wilkinson G; Wilson Z; Wood CM; Jewsbury PJ Discovery and Characterization of AZD6738, a Potent Inhibitor of Ataxia Telangiectasia Mutated and Rad3 Related (ATR) Kinase with Application as an Anticancer Agent. J. Med. Chem 2018, 61, 9889–9907. - PubMed
-
- Lücking U; Kosemund D; Böhnke N; Lienau P; Siemeister G; Denner K; Bohlmann R; Briem H; Terebesi I; Bömer U; Schäfer M; Ince S; Mumberg D; Scholz A; Izumi R; Hwang S; von Nussbaum F Changing for the Better: Discovery of the Highly Potent and Selective CDK9 Inhibitor VIP152 Suitable for Once Weekly Intravenous Dosing for the Treatment of Cancer. J. Med. Chem 2021, 64, 11651–11674. - PubMed
-
- Zhao P; Zeng Q Progress in the Enantioselective Synthesis of Sulfur (VI) Compounds. Chem. – Eur. J 2023, 29, No. e202302059. - PubMed
-
- Lücking U Neglected sulfur(vi) pharmacophores in drug discovery: exploration of novel chemical space by the interplay of drug design and method development. Org. Chem. Front 2019, 6, 1319–1324.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

