Drugs Targeting CD20 in Multiple Sclerosis: Pharmacology, Efficacy, Safety, and Tolerability
- PMID: 38480630
- PMCID: PMC10982103
- DOI: 10.1007/s40265-024-02011-w
Drugs Targeting CD20 in Multiple Sclerosis: Pharmacology, Efficacy, Safety, and Tolerability
Abstract
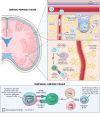
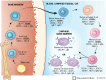
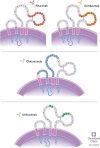
Currently, there are four monoclonal antibodies (mAbs) that target the cluster of differentiation (CD) 20 receptor available to treat multiple sclerosis (MS): rituximab, ocrelizumab, ofatumumab, and ublituximab. B-cell depletion therapy has changed the therapeutic landscape of MS through robust efficacy on clinical manifestations and MRI lesion activity, and the currently available anti-CD20 mAb therapies for use in MS are a cornerstone of highly effective disease-modifying treatment. Ocrelizumab is currently the only therapy with regulatory approval for primary progressive MS. There are currently few data regarding the relative efficacy of these therapies, though several clinical trials are ongoing. Safety concerns applicable to this class of therapeutics relate primarily to immunogenicity and mechanism of action, and include infusion-related or injection-related reactions, development of hypogammaglobulinemia (leading to increased infection and malignancy risk), and decreased vaccine response. Exploration of alternative dose/dosing schedules might be an effective strategy for mitigating these risks. Future development of biosimilar medications might make these therapies more readily available. Although anti-CD20 mAb therapies have led to significant improvements in disease outcomes, CNS-penetrant therapies are still needed to more effectively address the compartmentalized inflammation thought to play an important role in disability progression.
© 2024. The Author(s).
Conflict of interest statement
Alise K. Carlson reports personal compensation for consulting for Sanofi, Novartis, Bristol-Myers Squibb, and Vigil Neuro. Moein Amin reports fellowship grants from Novartis (NGC44741) and Biogen (23046PFEL). Jeffrey A. Cohen reports personal compensation for consulting for Astoria, Bristol-Myers Squibb, Convelo, EMD Serono, FiND Therapeutics, INMune, and Sandoz; and serving as an Editor of
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

