A Bursaphelenchus xylophilus effector BxICD1 inducing plant cell death, concurrently contributes to nematode virulence and migration
- PMID: 38481400
- PMCID: PMC10933036
- DOI: 10.3389/fpls.2024.1357141
A Bursaphelenchus xylophilus effector BxICD1 inducing plant cell death, concurrently contributes to nematode virulence and migration
Abstract
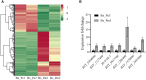
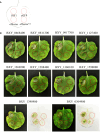
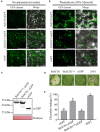
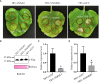
The migratory endoparasitic phytonematodes Bursaphelenchus xylophilus is the causal agent of pine wilt disease and causes significant economic damage to pine forests in China. Effectors play a key role in the successful parasitism of plants by phytonematodes. In this study, 210 genes obtained by transcriptomics analyses were found to be upregulated in B. xylophilus infecting Pinus massoniana that were not functionally annotated nor reported previously in B. xylophilus infecting P. thunbergii. Among these differentially expressed genes, a novel effector, BxICD1, that could induce cell death in the extracellular space of Nicotiana benthamiana was identified. BxICD1 was upregulated in the early stages of infection, as shown by RT-qPCR analyses. In situ hybridization analysis showed that BxICD1 was expressed in the esophageal gland of nematodes. The yeast signal sequence trap system indicated that BxICD1 possessed an N-terminal signal peptide with secretion functionality. Using an Agrobacterium-mediated transient expression system, it was demonstrated that the cell death-inducing activity of BxICD1 was dependent on N. benthamiana brassinosteroid-insensitive 1-associated kinase 1 (NbBAK1). Finally, BxICD1 contributed to B. xylophilus virulence and migration in host pine trees, as demonstrated by RNAi silencing assays. These findings indicate that BxICD1 both induces plant cell death and also contributes to nematode virulence and migration in P. massonian.
Keywords: Bursaphelenchus xylophilus; Pinus massoniana; effector; plant cell death activation; transcriptome.
Copyright © 2024 Li, Wang, Cao, Shan, He, Huang, Zhuo, Liao and Lin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc Series. B Stat. Methodol. 57, 289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x - DOI
-
- Cabasan M. T. N., Kumar A., Bellafiore S., De Waele D. (2014). Histopathology of the rice root-knot nematode, Meloidogyne graminicola, on Oryza sativa and O. glaberrima . Nematology 16, 73–81. doi: 10.1163/15685411-00002746 - DOI
-
- Chen J., Lin B., Huang Q., Hu L., Zhuo K., Liao J. (2017). A novel Meloidogyne graminicola effector, MgGPP, is secreted into host cells and undergoes glycosylation in concert with proteolysis to suppress plant defenses and promote parasitism. PLoS Pathog. 13, e1006301. doi: 10.1371/journal.ppat.1006301 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

