Hybrid Immunity Overcomes Defective Immune Response to COVID-19 Vaccination in Kidney Transplant Recipients
- PMID: 38481503
- PMCID: PMC10928013
- DOI: 10.1016/j.ekir.2023.12.008
Hybrid Immunity Overcomes Defective Immune Response to COVID-19 Vaccination in Kidney Transplant Recipients
Abstract
Introduction: Comorbidities and immunosuppressive therapies are associated with reduced immune responses to primary COVID-19 mRNA vaccination in kidney transplant recipients (KTRs). In healthy individuals, prior SARS-COV-2 infection is associated with increased vaccine responses, a phenotype called hybrid immunity. In this study, we explored the potential influence of immune suppression on hybrid immunity in KTRs.
Methods: Eighty-two KTRs, including 59 SARS-CoV-2-naïve (naïve KTRs [N-KTRs]) and 23 SARS-CoV-2-experienced (experienced KTRs [E-KTRs]) patients, were prospectively studied and compared to 106 healthy controls (HCs), including 40 SARS-CoV-2-naïve (N-HCs) and 66 SARS-CoV-2-experienced (E-HCs) subjects. Polyfunctional antibody and T cell responses were measured following 2 doses of BNT162b2 mRNA vaccine. Associations between vaccine responses and clinical characteristics were studied by univariate and multivariate analyses.
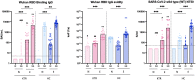
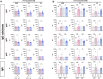
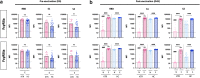
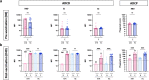
Results: In naïve KTRs, vaccine responses were markedly lower than in HCs and were correlated with older age, more recent transplantation, kidney retransplantation after graft failure, arterial hypertension, and treatment with mycophenolate mofetil (MMF). In contrast, vaccine responses of E-KTRs were similar to those of HCs and were associated with time between transplantation and vaccination, but not with the other risk factors associated with low vaccine responses in naïve KTRs.
Conclusion: In conclusion, hybrid immunity overcomes immune suppression and provides potent humoral and cellular immunity to SARS-CoV-2 in KTRs.
Keywords: COVID-19; hybrid immunity; kidney transplantation; mRNA vaccination; nonneutralizing antibodies; systems immunology.
© 2023 International Society of Nephrology. Published by Elsevier Inc.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous

