Tumor-derived apoptotic extracellular vesicle-mediated intercellular communication promotes metastasis and stemness of lung adenocarcinoma
- PMID: 38481566
- PMCID: PMC10933389
- DOI: 10.1016/j.bioactmat.2024.02.026
Tumor-derived apoptotic extracellular vesicle-mediated intercellular communication promotes metastasis and stemness of lung adenocarcinoma
Abstract
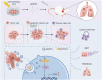
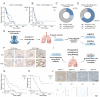
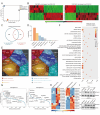
Apoptosis has long been recognized as a significant mechanism for inhibiting tumor formation, and a plethora of stimuli can induce apoptosis during the progression and treatment of tumors. Moreover, tumor-derived apoptotic extracellular vesicles (apoEVs) are inevitably phagocytosed by live tumor cells, promoting tumor heterogeneity. Understanding the mechanism by which apoEVs regulate tumor cells is imperative for enhancing our knowledge of tumor metastasis and recurrence. Herein, we conducted a series of in vivo and in vitro experiments, and we report that tumor-derived apoEVs promoted lung adenocarcinoma (LUAD) metastasis, self-renewal and chemoresistance. Mechanistically, we demonstrated that apoEVs facilitated tumor metastasis and stemness by initiating the epithelial-mesenchymal transition program and upregulating the transcription of the stem cell factor SOX2. In addition, we found that ALDH1A1, which was transported by apoEVs, activated the NF-κB signaling pathway by increasing aldehyde dehydrogenase enzyme activity in recipient tumor cells. Furthermore, targeting apoEVs-ALDH1A1 significantly abrogated these effects. Collectively, our findings elucidate a novel mechanism of apoEV-dependent intercellular communication between apoptotic tumor cells and live tumor cells that promotes the formation of cancer stem cell-like populations, and these findings reveal that apoEVs-ALDH1A1 may be a potential therapeutic target and biomarker for LUAD metastasis and recurrence.
Keywords: Apoptotic extracellular vesicles; Lung adenocarcinoma; Proteomics; SOX2; Stemness.
© 2024 The Authors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Apoptotic cell-derived extracellular vesicles-MTA1 confer radioresistance in cervical cancer by inducing cellular dormancy.J Transl Med. 2025 Mar 14;23(1):328. doi: 10.1186/s12967-025-06350-4. J Transl Med. 2025. PMID: 40087679 Free PMC article.
-
Extracellular vesicles from the dead: the final message.Trends Cell Biol. 2025 May;35(5):439-452. doi: 10.1016/j.tcb.2024.09.005. Epub 2024 Oct 21. Trends Cell Biol. 2025. PMID: 39438206 Review.
-
Apoptotic extracellular vesicles derived from hypoxia-preconditioned mesenchymal stem cells within a modified gelatine hydrogel promote osteochondral regeneration by enhancing stem cell activity and regulating immunity.J Nanobiotechnology. 2024 Feb 23;22(1):74. doi: 10.1186/s12951-024-02333-7. J Nanobiotechnology. 2024. PMID: 38395929 Free PMC article.
-
Apoptotic extracellular vesicles alleviate Pg-LPS induced inflammatory responses of macrophages via AMPK/SIRT1/NF-κB pathway and inhibit osteoclast formation.J Periodontol. 2022 Nov;93(11):1738-1751. doi: 10.1002/JPER.21-0657. Epub 2022 Aug 4. J Periodontol. 2022. PMID: 35499816
-
Mesenchymal Stem Cells-Derived Apoptotic Extracellular Vesicles (ApoEVs): Mechanism and Application in Tissue Regeneration.Stem Cells. 2023 Sep 15;41(9):837-849. doi: 10.1093/stmcls/sxad046. Stem Cells. 2023. PMID: 37338056 Review.
Cited by
-
Rapid and unbiased enrichment of extracellular vesicles via a meticulously engineered peptide.Bioact Mater. 2024 Sep 26;43:292-304. doi: 10.1016/j.bioactmat.2024.09.023. eCollection 2025 Jan. Bioact Mater. 2024. PMID: 39399836 Free PMC article.
-
TNFAIP2 promotes NF-κB signaling mediate lymph node metastasis of oral squamous cell carcinoma by protecting IKKβ from ubiquitin proteasome degradation.Cell Commun Signal. 2025 Feb 13;23(1):83. doi: 10.1186/s12964-025-02077-3. Cell Commun Signal. 2025. PMID: 39948570 Free PMC article.
-
Detection of Cancer Stem Cells from Patient Samples.Cells. 2025 Jan 20;14(2):148. doi: 10.3390/cells14020148. Cells. 2025. PMID: 39851576 Free PMC article. Review.
-
ApoBDs: a paradigm shift from cellular debris to therapeutic vehicles.Front Endocrinol (Lausanne). 2025 Jul 17;16:1626796. doi: 10.3389/fendo.2025.1626796. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40747304 Free PMC article. Review.
-
Chemotherapy reprograms miRNA expression profiles in apoptotic extracellular vesicles from medulloblastoma cells, regulating pro- and anti-proliferative effects on recipient drug-naïve cells.Cell Commun Signal. 2025 Jun 10;23(1):273. doi: 10.1186/s12964-025-02241-9. Cell Commun Signal. 2025. PMID: 40495204 Free PMC article.
References
-
- Travis W.D., Brambilla E., Noguchi M., Nicholson A.G., Geisinger K.R., Yatabe Y., Beer D.G., Powell C.A., Riely G.J., Van Schil P.E., Garg K., Austin J.H.M., Asamura H., Rusch V.W., Hirsch F.R., Scagliotti G., Mitsudomi T., Huber R.M., Ishikawa Y., Jett J., Sanchez-Cespedes M., Sculier J.-P., Takahashi T., Tsuboi M., Vansteenkiste J., Wistuba I., Yang P.-C., Aberle D., Brambilla C., Flieder D., Franklin W., Gazdar A., Gould M., Hasleton P., Henderson D., Johnson B., Johnson D., Kerr K., Kuriyama K., Lee J.S., Miller V.A., Petersen I., Roggli V., Rosell R., Saijo N., Thunnissen E., Tsao M., Yankelewitz D. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J. Thorac. Oncol. 2011;6:244–285. doi: 10.1097/JTO.0b013e318206a221. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

