Inversion of the Warburg Effect: Unraveling the Metabolic Nexus between Obesity and Cancer
- PMID: 38481689
- PMCID: PMC10928896
- DOI: 10.1021/acsptsci.3c00301
Inversion of the Warburg Effect: Unraveling the Metabolic Nexus between Obesity and Cancer
Abstract
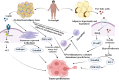
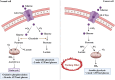
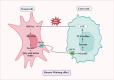
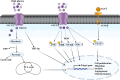
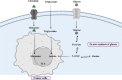
Obesity is a well-established risk factor for cancer, significantly impacting both cancer incidence and mortality. However, the intricate molecular mechanisms connecting adipose tissue to cancer cell metabolism are not fully understood. This Review explores the historical context of tumor energy metabolism research, tracing its origins to Otto Warburg's pioneering work in 1920. Warburg's discovery of the "Warburg effect", wherein cancer cells prefer anaerobic glycolysis even in the presence of oxygen, laid the foundation for understanding cancer metabolism. Building upon this foundation, the "reverse Warburg effect" emerged in 2009, elucidating the role of aerobic glycolysis in cancer-associated fibroblasts (CAFs) and its contribution to lactate accumulation in the tumor microenvironment, subsequently serving as a metabolic substrate for cancer cells. In contrast, within high-adiposity contexts, cancer cells exhibit a unique metabolic shift termed the "inversion of the Warburg effect". This phenomenon, distinct from the stromal-dependent reverse Warburg effect, relies on increased nutrient abundance in obesity environments, leading to the generation of glucose from lactate as a metabolic substrate. This Review underscores the heightened tumor proliferation and aggressiveness associated with obesity, introducing the "inversion of the Warburg effect" as a novel mechanism rooted in the altered metabolic landscape within an obese milieu. The insights presented here open promising avenues for therapeutic exploration, offering fresh perspectives and opportunities for the development of innovative cancer treatment strategies.
© 2024 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Lobstein T.; Brinsden H.; Neveux M.. World Obesity Atlas 2022; World Obesity Federation, March 2022. https://www.worldobesity.org/resources/resource-library/world-obesity-at...
Publication types
LinkOut - more resources
Full Text Sources
