NK-92MI Cells Engineered with Anti-claudin-6 Chimeric Antigen Receptors in Immunotherapy for Ovarian Cancer
- PMID: 38481806
- PMCID: PMC10929190
- DOI: 10.7150/ijbs.88539
NK-92MI Cells Engineered with Anti-claudin-6 Chimeric Antigen Receptors in Immunotherapy for Ovarian Cancer
Abstract
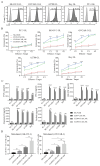
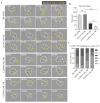
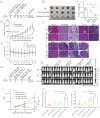
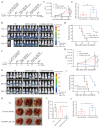
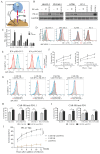
Background: The application of chimeric antigen receptor (CAR) NK cells in solid tumors is hindered by lack of tumor-specific targets and inefficient CAR-NK cell efficacy. Claudin-6 (CLDN6) has been reported to be overexpressed in ovarian cancer and may be an attractive target for CAR-NK cells immunotherapy. However, the feasibility of using anti-CLDN6 CAR-NK cells to treat ovarian cancer remains to be explored. Methods: CLDN6 expression in primary human ovarian cancer, normal tissues and cell lines were detected by immunohistochemistry and western blot. Two types of third-generation CAR NK-92MI cells targeting CLDN6, CLDN6-CAR1 NK-92MI cells with domains containing self-activated elements (NKG2D, 2B4) and CLDN6-CAR2 NK-92MI cells with classical domains (CD28, 4-1BB) were constructed by lentivirus transfection, sorted by flow cytometry and verified by western blot and qPCR. OVCAR-3, SK-OV-3, A2780, Hey and PC-3 cells expressing the GFP and luciferase genes were transduced. Subcutaneous and intraperitoneal tumor models were established via NSG mice. The ability of CLDN6-CAR NK cells to kill CLDN6-positive ovarian cancer cells were evaluated in vitro and in vivo by live cell imaging and bioluminescence imaging. Results: Both CLDN6-CAR1 and CLDN6-CAR2 NK-92MI cells could specifically killed CLDN6-positive ovarian cancer cells (OVCAR-3, SK-OV-3, A2780 and Hey), rather than CLDN6 negative cell (PC-3), in vitro. CLDN6-CAR1 NK-92MI cells with domains containing self-activated elements (NKG2D, 2B4) exhibited stronger cytotoxicity than CLDN6-CAR2 NK-92MI cells with classical domains (CD28, 4-1BB). Furthermore, CLDN6-CAR1 NK cells could effectively eliminate ovarian cancer cells in subcutaneous and intraperitoneal tumor models. More importantly, CAR-NK cells combined with immune checkpoint inhibitors, anti-PD-L1, could synergistically enhance the antitumor efficacy of CLDN6-targeted CAR-NK cells. Conclusions: These results indicate that CLDN6-CAR NK cells possess strong antitumor activity and represent a promising immunotherapeutic modality for ovarian cancer.
Keywords: Claudin-6; NK cells; PD-1; PD-L1; chimeric antigen receptor; ovarian cancer.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


References
-
- Henderson JT, Webber EM, Sawaya GF. Screening for Ovarian Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2018;319(6):595–606. - PubMed
-
- Lorusso D, Ceni V, Muratore M. et al. Emerging role of immune checkpoint inhibitors in the treatment of ovarian cancer. Expert Opin Emerg Drugs. 2020;25(4):445–453. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

