Innate Immune Training in Chickens for Improved Defense against Pathogens: A Review
- PMID: 38481975
- PMCID: PMC10925860
- DOI: 10.2141/jpsa.2024008
Innate Immune Training in Chickens for Improved Defense against Pathogens: A Review
Abstract
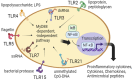
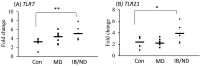
The avian immune system plays a vital role in poultry production to obtain good productibility and products that are safe and of high quality. Historically, adaptive immunity has been the main target of vaccination. However, over the past decade, innate immunity has been reported to be enhanced in different animals through vaccination and feed additives. This enhancement is due to innate immune memory termed "trained immunity," in which epigenetic and metabolic reprogramming play significant roles. Although reports on trained immunity in poultry are limited, several studies have suggested that vaccinations and feed additives affect the innate immunity. This review discusses the possible effects of vaccination and β-glucan on innate immunity for potential incorporation in advanced strategies to enhance the defense function in poultry while considering the information on trained immunity in mammals.
Keywords: innate immunity; reprograming; trained immunity; vaccines; β-glucan.
2024 Japan Poultry Science Association.
Conflict of interest statement
Conflicts of Interest: The authors declare no conflict of interest.
Figures


References
-
- Schijns V,Sharma J andTarpey I. Practical aspects of poultry vaccination. In: Avian Immunology (Davison F, Kaspers B and Schat K eds.), Elsevier Ltd, London, pp. 373-394, 2008.
-
- Kaufman J. The avian major histocompatibility complex. In: Avian Immunology (Kaspers B, Schat K, Göbel T, Vervelde L eds), 3ird ed, Elsevier Ltd, London, pp. 135-161, 2022.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

