The Role and Limitations of the Reference Interval Within Clinical Chemistry and Its Reliability for Disease Detection
- PMID: 38481978
- PMCID: PMC10932992
- DOI: 10.3389/bjbs.2024.12339
The Role and Limitations of the Reference Interval Within Clinical Chemistry and Its Reliability for Disease Detection
Abstract
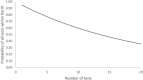
Reference intervals (RIs) are a range of values that are supplied alongside laboratory measurements for comparison to allow interpretation of this data. Historically, RIs were referred to as the normal range. However, the perception of what is normal can lead to confusion in clinicians and unnecessary emotional distress in patients. RIs can be acquired using several methods. Laboratories may quote published studies or derive their own using established direct or indirect methods. Alternatively, laboratories may verify RIs provided by assay manufacturers using in-house studies. RIs have several limitations that clinicians should be aware of. The statistical methodology associated with establishment of RIs means that approximately 5% of "disease free" individuals will fall outside the RI. Additionally, the higher the number of tests requested, the higher the probability that one will be abnormal, and repeat results in an individual may show regression to the mean. Completion of studies for establishment of RIs can be expensive, difficult, and time consuming. Method bias and differences in populations can greatly influence RIs and prevent them from being transferable between some laboratories. Differences in individual characteristics such as age, ethnicity, and sex can result in large variation in some analytes. Some patients, such as those whose gender differs from that which was presumed for them at birth, may require their own RIs. Alternatively, a decision will need to be made about which to use. Overall, the issue common to these factors lies within interpretation. As such, RIs can be improved with better training in their use, combined with a better understanding of influences that affect them, and more transparent communication from laboratories in how RIs were derived.
Keywords: clinical chemistry; clinical decision curve; normal range; reference intervals; reference ranges.
Copyright © 2024 Timbrell.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Fisher R. Statistical Methods for Research Workers. 5th ed. Edinburgh: Oliver & Boyd; (1934).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials