Influence of arylalkyl amines on the formation of hybrid CsPbBr3 nanocrystals via a modified LARP method
- PMID: 38482026
- PMCID: PMC10929594
- DOI: 10.1039/d3na01105d
Influence of arylalkyl amines on the formation of hybrid CsPbBr3 nanocrystals via a modified LARP method
Abstract
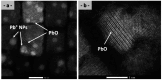
Perovskite nanocrystals have attracted much attention in the last ten years due to their different applications, especially in the photovoltaic domain and LED performance. In this large family of perovskite nanocrystals, CsPbBr3 nanocrystals are attractive nanomaterials because they are good candidates for obtaining green emissions and exploring new synthesis routes. In this context, controlling the nanometric scale's morphology, particularly the size and monodispersity, is fundamental for exploring their photophysical properties and final applications. Currently, the nanometric size of nanocrystals is ensured by the presence of oleic acid and oleylamine molecules, in using Hot Injection (HI) or ligand-assisted reprecipitation (LARP) methods. If oleic acid plays a fundamental role, oleylamine can be easily substituted by other amino molecules, opening the way for the functionalization of CsPbBr3 nanocrystals and the obtention of new hybrid perovskite nanocrystal families. In this article, we describe the synthesis, by soft chemistry, of a new family of hybrid organic-inorganic CsPbBr3 nanocrystals, functionalized by aryl-alkylamine (AAA) molecules, through the modified LARP method. We highlight the mechanism for cutting submicron crystals into nanocrystals, using aryl-alkylamine molecules like scissors. The impact of these amino molecules on the final nanocrystals leads to different nanocrystal morphologies (nanocubes, nanosheets, or nanorods) and structures (monoclinic, rhombohedral, or tetragonal). In addition, this modified LARP method highlights, under certain experimental conditions, an unexpected formation of PbO ribbons.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
6There are no conflicts to declare.
Figures

















References
-
- Huang Q. J. Zhu Y. Adv. Mater. Technol. 2019;4:1800546. doi: 10.1002/admt.201800546. - DOI
-
- Wang Y. Song L. Chen Y. Huang W. ACS Photonics. 2020;7:10–28. doi: 10.1021/acsphotonics.9b01233. - DOI
-
- Shen W. Chen J. Wu J. Li X. Zeng H. ACS Photonics. 2021;8:113–124. doi: 10.1021/acsphotonics.0c01501. - DOI
LinkOut - more resources
Full Text Sources

