Comparative pathogenicity of CA1737/04 and Mass infectious bronchitis virus genotypes in laying chickens
- PMID: 38482170
- PMCID: PMC10932974
- DOI: 10.3389/fvets.2024.1338563
Comparative pathogenicity of CA1737/04 and Mass infectious bronchitis virus genotypes in laying chickens
Abstract
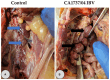
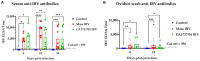
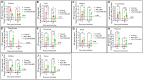
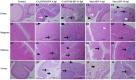
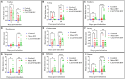
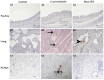
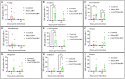
Infectious bronchitis virus (IBV) is a respiratory virus causing atropism in multiple body systems of chickens. Recently, the California 1737/04 (CA1737/04) IBV strain was identified as one of the circulating IBV variants among poultry operations in North America. Here, the pathogenicity and tissue tropism of CA1737/04 IBV strain in specific-pathogen-free (SPF) hens were characterized in comparison to Massachusetts (Mass) IBV. In 30 weeks-old SPF hens, Mass or CA1737/04 IBV infections were carried out, while the third group was maintained as a control group. Following infection, we evaluated clinical signs, egg production, viral shedding, serology, necropsy examination, and histopathology during a period of 19 days. Also, certain tissue affinity parameters were investigated, which involved the localization of viral antigens and the detection of viral RNA copies in designated tissues. Our findings indicate that infection with CA1737/04 or Mass IBV strain could induce significant clinical signs, reduced egg production, and anti-IBV antibodies locally in oviduct wash and systemically in serum. Both IBV strains showed detectable levels of viral RNA copies and induced pathology in respiratory, renal, enteric, and reproductive tissues. However, the CA1737/04 IBV strain had higher pathogenicity, higher tissue tropism, and higher replication in the kidney, large intestine, and different segments of the oviduct compared to the Mass IBV strain. Both IBV strains shed viral genome from the cloacal route, however, the Mass IBV infected hens shed higher IBV genome loads via the oropharyngeal route compared to CA1737/04 IBV-infected hens. Overall, the current findings could contribute to a better understanding of CA1737/04 IBV pathogenicity in laying hens.
Keywords: Canada; hen; infectious bronchitis virus; pathogenicity; tissue tropism.
Copyright © 2024 Ali, Farooq, Altakrouni, Najimudeen, Hassan, Isham, Shalaby, Gallardo and Abdul-Careem.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
Comparative pathogenicity of infectious bronchitis virus Massachusetts and Delmarva (DMV/1639) genotypes in laying hens.Front Vet Sci. 2024 Jan 19;10:1329430. doi: 10.3389/fvets.2023.1329430. eCollection 2023. Front Vet Sci. 2024. PMID: 38313768 Free PMC article.
-
Pathogenicity comparison between QX-type and Mass-type infectious bronchitis virus to different segments of the oviducts in laying phase.Virol J. 2022 Apr 7;19(1):62. doi: 10.1186/s12985-022-01788-0. Virol J. 2022. PMID: 35392927 Free PMC article.
-
Efficacy of Two Vaccination Strategies against Infectious Bronchitis in Laying Hens.Vaccines (Basel). 2023 Feb 2;11(2):338. doi: 10.3390/vaccines11020338. Vaccines (Basel). 2023. PMID: 36851216 Free PMC article.
-
Avian infectious bronchitis virus.Rev Sci Tech. 2000 Aug;19(2):493-508. doi: 10.20506/rst.19.2.1228. Rev Sci Tech. 2000. PMID: 10935276 Review.
-
Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus.Avian Pathol. 2003 Dec;32(6):567-82. doi: 10.1080/03079450310001621198. Avian Pathol. 2003. PMID: 14676007 Free PMC article. Review.
Cited by
-
Genetic and Phenotypic Investigations of Viral Subpopulations Detected in Different Tissues of Laying Hens Following Infectious Bronchitis Virus Infection.Viruses. 2025 Apr 4;17(4):527. doi: 10.3390/v17040527. Viruses. 2025. PMID: 40284970 Free PMC article.
References
-
- Amarasinghe A, Popowich S, De Silva Senapathi U, Abdul-Cader MS, Marshall F, van der Meer F, et al. . Shell-less egg syndrome (SES) widespread in western Canadian layer operations is linked to a Massachusetts (Mass) type infectious bronchitis virus (IBV) isolate. Viruses. (2018) 10:437. doi: 10.3390/v10080437, PMID: - DOI - PMC - PubMed
-
- Reddy VR, Trus I, Desmarets LM, Li Y, Theuns S, Nauwynck HJ. Productive replication of nephropathogenic infectious bronchitis virus in peripheral blood monocytic cells, a strategy for viral dissemination and kidney infection in chickens. Vet Res. (2016) 47:70. doi: 10.1186/s13567-016-0354-9, PMID: - DOI - PMC - PubMed

