Immunotherapy-associated cardiovascular toxicities: insights from preclinical and clinical studies
- PMID: 38482205
- PMCID: PMC10932998
- DOI: 10.3389/fonc.2024.1347140
Immunotherapy-associated cardiovascular toxicities: insights from preclinical and clinical studies
Abstract
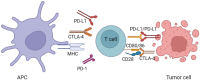
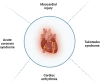
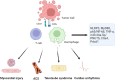
Immune checkpoint inhibitors (ICIs) have become a widely accepted and effective treatment for various types of solid tumors. Recent studies suggest that cardiovascular immune-related adverse events (irAEs) specifically have an incidence rate ranging from 1.14% to more than 5%. Myocarditis is the most common observed cardiovascular irAE. Others include arrhythmias, pericardial diseases, vasculitis, and a condition resembling takotsubo cardiomyopathy. Programmed cell death-1 (PD-1)/programmed cell death ligand-1 (PD-L1) pathway, cytotoxic T-lymphocyte antigen-4 (CTLA-4) pathway, and the recently discovered lymphocyte-activation gene 3 (LAG-3) pathway, play a critical role in boosting the body's natural immune response against cancer cells. While ICIs offer significant benefits in terms of augmenting immune function, they can also give rise to unwanted inflammatory side effects known as irAEs. The occurrence of irAEs can vary in severity, ranging from mild to severe, and can impact the overall clinical efficacy of these agents. This review aims to summarize the underlying mechanisms of cardiovascular irAE from both preclinical and clinical studies for a better understanding of cardiovascular irAE in clinical application.
Keywords: cardio-oncology; clinical studies; immune checkpoint inhibitors; immune-related adverse events; preclinical studies.
Copyright © 2024 Kong, Wang and Qie.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials