Circulating tumor DNA analysis guiding adjuvant treatment in resected stage III cholangiocarcinoma: a case report
- PMID: 38482231
- PMCID: PMC10932665
- DOI: 10.21037/jgo-23-815
Circulating tumor DNA analysis guiding adjuvant treatment in resected stage III cholangiocarcinoma: a case report
Abstract
Background: Cholangiocarcinoma (CCA) is a rare and aggressive gastrointestinal cancer. Unfortunately, 60% to 70% of early-stage CCA patients experience disease recurrence after curative resection and standard adjuvant therapy. Currently, there is no reliable tool to identify CCA recurrence before radiographic detection. Longitudinal monitoring of circulating tumor DNA (ctDNA) has shown promising value in molecular identification of relapse prior to conventional surveillance in other solid tumors. However, there is a scarcity of data on ctDNA in CCA after curative surgery.
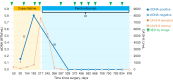
Case description: An 81-year-old male with stage 3A intrahepatic CCA achieved radiographic remission after curative resection and was started on standard adjuvant capecitabine on post-operative day (POD) 50. Tumor-informed ctDNA tested positive on two consecutive occasions, with the titer increasing from 0.16 mean tumor molecule (MTM)/mL on POD 92 to 0.80 MTM/mL on POD 183, despite being on capecitabine. carbohydrate antigen 19-9 (CA19-9) also continued to increase from 175.6 U/mL on POD 92 to 7,594.9 U/mL on POD 217. Notably, surveillance computed tomography (CT) scans showed no evidence of disease (NED) on POD 126, 186, and 211. Molecular profiling and next-generation sequencing (NGS) panels from CCA tissue revealed microsatellite instability-high (MSI-H). After extensive discussions with the patient regarding the rising ctDNA titer despite being on capecitabine for nearly 6 months, we initiated pembrolizumab on POD 224 prior to radiographic recurrence. Given the tumor is MSI-H, and the preferred toxicity profile compared to the front-line chemotherapy option for CCA, we started pembrolizumab. ctDNA became undetectable, and CA19-9 returned to the reference range with pembrolizumab. As of the last follow-up on POD 876, the patient has continued pembrolizumab without noticeable side effects, and imaging continues to show NED, with persistent negative ctDNA and normal CA19-9 levels.
Conclusions: This case demonstrates the potential utility of tumor-informed ctDNA in CCA as (I) an early detection tool before radiographic recurrence; (II) a response monitoring tool as a surrogate biomarker that can guide therapy optimization; and (III) shows that early intervention with immunotherapy or potentially targeted agents based on ctDNA may lead to improved survival outcomes.
Keywords: Cholangiocarcinoma (CCA); case report; circulating tumor DNA (ctDNA); ctDNA-guided therapy.
2024 Journal of Gastrointestinal Oncology. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-815/coif). R.D.K. reports consulting/advisory fees from AstraZeneca, Exelixis, Ipsen, Eisai, Roche, and Pfizer and Speakers Bureau fees from Incyte and AstraZeneca. C.M.R. reports Speakers Bureau fees from AstraZeneca. The other authors have no conflicts of interest to declare.
Figures
Comment in
-
Emerging role of circulating tumor DNA for early detection of recurrence in biliary tract cancers.J Gastrointest Oncol. 2024 Jun 30;15(3):1358-1362. doi: 10.21037/jgo-24-224. Epub 2024 Apr 25. J Gastrointest Oncol. 2024. PMID: 38989432 Free PMC article. No abstract available.
References
-
- Oh DY, He AR, Qin S, et al. 78P Updated overall survival (OS) from the phase III TOPAZ-1 study of durvalumab (D) or placebo (PBO) plus gemcitabine and cisplatin (+ GC) in patients (pts) with advanced biliary tract cancer (BTC). Ann Oncol 2022;33:S1462-3. 10.1016/j.annonc.2022.10.114 - DOI
-
- Kelley RK, Ueno M, Yoo C, et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023;401:1853-65. 10.1016/S0140-6736(23)00727-4 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
