Decisions of the FDA on premarket tobacco product applications: Changes in the number of unique devices and liquids used by US adults who frequently use electronic nicotine delivery systems, 2020-2023
- PMID: 38482508
- PMCID: PMC10936557
- DOI: 10.18332/tid/184240
Decisions of the FDA on premarket tobacco product applications: Changes in the number of unique devices and liquids used by US adults who frequently use electronic nicotine delivery systems, 2020-2023
Abstract
Introduction: The majority of decisions on electronic nicotine delivery system (ENDS) premarket tobacco product applications (PMTAs) were made from October 2020 to February 2023; 99% (>25 million) had determinations by March 2023 and just twenty-three received marketing granted orders. We examined the unique devices and liquids used among US adults frequently using ENDS before, during, and after a majority of PMTA decisions were made.
Methods: Data are from waves 1-5 (W1: May-Oct 2020, n=1179; W5: Feb-Apr 2023, n=1290) of a longitudinal survey of US adults (≥21 years) using ENDS ≥5 days/week. User-submitted photos of participants' most used devices and liquids were coded. Descriptive analyses and Wilcoxon signed-rank tests were used to understand the number and types of unique devices and liquids used in W1-W5, and the top brands in each wave.
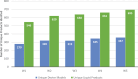
Results: From W1-W5, the number of unique ENDS device models and liquid products used by participants increased from 279 to 357 (p<0.001) and 546 to 695 (p<0.001), respectively. More unique devices in W5 versus W1 were disposable (W1: 16.5%; W5: 36.1%); fewer were disposable pod (W1: 6.5%; W5: 3.1%) or tank (W1: 53.8%; W5: 30.8%) devices. Liquids were primarily sweet-flavored (W1: 81.1%; W5: 82.0%). The median liquid nicotine concentration increased from 12 to 50 mg/mL. In W5, few participants used FDA-approved devices (n=17; 1.3%) or liquids (n=6; 0.5%), and Elf Bar was the most commonly used device and liquid brand. Results for all waves are reported.
Conclusions: Despite PMTA decisions, an increase in the number of unique device models and liquid products used among adults who frequently use ENDS was observed from 2020 to 2023. Few participants in 2023 were using FDA-approved devices or liquids. Further research and monitoring are needed to inform how FDA prioritizes enforcement actions and what types of enforcement actions are effective.
Keywords: ENDS; policy; regulatory science; survey; tobacco.
© 2024 Crespi E. et al.
Conflict of interest statement
The authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors declare that they have no competing interests, financial or otherwise, related to the current work. All authors report that since the initial planning of the work, this study was supported by the NIH National Institute on Drug Abuse and FDA Center for Tobacco Products. J. E. Cohen reports that in the past 36 months, she has been a paid consultant in litigation against a tobacco company. She is also a member of the Board of the Society for Research on Nicotine and Tobacco.
Figures
References
-
- Gravely S, Driezen P, Ouimet J, et al. . Prevalence of awareness, ever-use and current use of nicotine vaping products (NVPs) among adult current smokers and ex-smokers in 14 countries with differing regulations on sales and marketing of NVPs: cross-sectional findings from the ITC Project. Addiction. 2019;114(6):1060-1073. doi:10.1111/add.14558 - DOI - PMC - PubMed
-
- Food and Drug Administration . Premarket Tobacco Product Applications. Accessed February 20, 2024. https://www.fda.gov/tobacco-products/market-and-distribute-tobacco-produ...
-
- Food and Drug Administration . Marketing Orders for SE. January 29, 2024. Accessed February 20, 2024. https://www.fda.gov/tobacco-products/substantial-equivalence/marketing-o...
-
- Food and Drug Administration . PMTA Acceptance Phase Metrics. February 28, 2023. Accessed February 20, 2024. https://www.fda.gov/media/167416/download
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous