Mice with a Pax2 missense variant display impaired glomerular repair
- PMID: 38482556
- PMCID: PMC12040299
- DOI: 10.1152/ajprenal.00259.2023
Mice with a Pax2 missense variant display impaired glomerular repair
Abstract
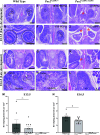
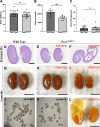
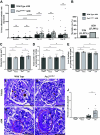
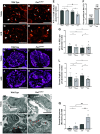
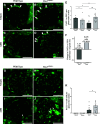
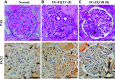
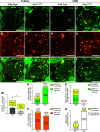
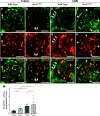
PAX2 regulates kidney development, and its expression persists in parietal epithelial cells (PECs), potentially serving as a podocyte reserve. We hypothesized that mice with a Pax2 pathogenic missense variant (Pax2A220G/+) have impaired PEC-mediated podocyte regeneration. Embryonic wild-type mouse kidneys showed overlapping expression of PAX2/Wilms' tumor-1 (WT-1) until PEC and podocyte differentiation, reflecting a close lineage relationship. Embryonic and adult Pax2A220G/+ mice have reduced nephron number but demonstrated no glomerular disease under baseline conditions. Pax2A220G/+ mice compared with wild-type mice were more susceptible to glomerular disease after adriamycin (ADR)-induced podocyte injury, as demonstrated by worsened glomerular scarring, increased podocyte foot process effacement, and podocyte loss. There was a decrease in PAX2-expressing PECs in wild-type mice after adriamycin injury accompanied by the occurrence of PAX2/WT-1-coexpressing glomerular tuft cells. In contrast, Pax2A220G/+ mice showed no changes in the numbers of PAX2-expressing PECs after adriamycin injury, associated with fewer PAX2/WT-1-coexpressing glomerular tuft cells compared with injured wild-type mice. A subset of PAX2-expressing glomerular tuft cells after adriamycin injury was increased in Pax2A220G/+ mice, suggesting a pathological process given the worse outcomes observed in this group. Finally, Pax2A220G/+ mice have increased numbers of glomerular tuft cells expressing Ki-67 and cleaved caspase-3 compared with wild-type mice after adriamycin injury, consistent with maladaptive responses to podocyte loss. Collectively, our results suggest that decreased glomerular numbers in Pax2A220G/+ mice are likely compounded with the inability of their mutated PECs to regenerate podocyte loss, and together these two mechanisms drive the worsened focal segmental glomerular sclerosis phenotype in these mice.NEW & NOTEWORTHY Congenital anomalies of the kidney and urinary tract comprise some of the leading causes of kidney failure in children, but our previous study showed that one of its genetic causes, PAX2, is also associated with adult-onset focal segmental glomerular sclerosis. Using a clinically relevant model, our present study demonstrated that after podocyte injury, parietal epithelial cells expressing PAX2 are deployed into the glomerular tuft to assist in repair in wild-type mice, but this mechanism is impaired in Pax2A220G/+ mice.
Keywords: PAX2; glomerular repair; parietal epithelial cells; podocyte; regeneration.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


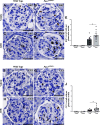
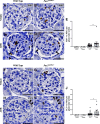
References
-
- Verberne WR, Das-Gupta Z, Allegretti AS, Bart HAJ, van Biesen W, García-García G, Gibbons E, Parra E, Hemmelder MH, Jager KJ, Ketteler M, Roberts C, Al Rohani M, Salt MJ, Stopper A, Terkivatan T, Tuttle KR, Yang C-W, Wheeler DC, Bos WJW. Development of an international standard set of value-based outcome measures for patients with chronic kidney disease: a report of the International Consortium for Health Outcomes Measurement (ICHOM) CKD Working Group. Am J Kidney Dis 73: 372–384, 2019. doi:10.1053/j.ajkd.2018.10.007. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

