TROP-2, NECTIN-4 and predictive biomarkers in sarcomatoid and rhabdoid bladder urothelial carcinoma
- PMID: 38482675
- PMCID: PMC10938277
- DOI: 10.32074/1591-951X-937
TROP-2, NECTIN-4 and predictive biomarkers in sarcomatoid and rhabdoid bladder urothelial carcinoma
Abstract
Introduction: The surface protein TROP-2/TACSTD2 and the cell adhesion protein NECTIN-4/NECTIN4 are responsible for the efficacy of anticancer therapies based on antibody-drug conjugates (ADC) targeting intracellular microtubules. In contrast with common histologic subtypes of bladder urothelial carcinoma (BUC), little is known of TROP-2 and NECTIN-4 expression in sarcomatoid and rhabdoid BUC.
Aims: In this study, we aimed to analyze TROP-2 and NECTIN-4 expression and additional predictive biomarkers by immunohistochemistry and fluorescence in situ hybridization (FISH) on 35 undifferentiated BUC (28 sarcomatoid and 7 rhabdoid). Wide genomic investigation was also performed on 411 BUC cases of the PanCancer Atlas, focusing on genes related to the microtubule pathways.
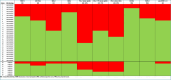
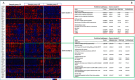
Results: Seven of 35 (20%) undifferentiated BUC showed expression of TROP-2. NECTIN-4 was expressed in 10 cases (29%). Seven cases (20%) co-expressed TROP-2 and NECTIN-4. HER-2 FISH was amplified in 5 cases (14%) while HER-2 immunoexpression was observed in 14 cases (40%). PD-L1 scored positive for combined proportion score (CPS) in 66% of cases and for tumor proportion score (TPS) in 51% of cases. Pan-NTRK1-2/3 was elevated in 9 cases (26%) and FGFR-2/3 was broken in 7 of 35 cases (20%). Of 28 sarcomatoid BUC, 9 (32%) were negative for all (TROP-2, NECTIN-4, PD-L1, HER-2, FGFR and pan-NTRK) biomarkers and 3 (11%) expressed all five biomarkers. Among cases with rhabdoid dedifferentiation, 1 of 7 (14%) showed activation of all biomarkers, whereas 2 of 7 (28%) showed none. The mRNA analysis identified microtubule-related genes and pathways suitable for combined ADC treatments in BUC.
Conclusion: Sarcomatoid and rhabdoid BUC do harbor positive expression of the ADC targets TROP-2 or NECTIN-4 in a relatively modest subset of cases, whereas the majority do not. Different combinations of other positive biomarkers may help the choice of medical therapies. Overall, these findings have important clinical implications for targeted therapy for BUC.
Keywords: NECTIN-4; TROP-2; biomarker; bladder; urothelial carcinoma.
Copyright © 2024 Società Italiana di Anatomia Patologica e Citopatologia Diagnostica, Divisione Italiana della International Academy of Pathology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Mollica V, Rizzo A, Montironi R, et al. . Current Strategies and Novel Therapeutic Approaches for Metastatic Urothelial Carcinoma. Cancers. 2020;12:1449. https://doi.org/10.3390/cancers12061449 10.3390/cancers12061449 - DOI - PMC - PubMed
-
- Chou J, Trepka K, Sjöström M, et al. . TROP2 Expression Across Molecular Subtypes of Urothelial Carcinoma and Enfortumab Vedotin-resistant Cells. Eur Urol Oncol. 2022;5:714-718. https://doi.org/10.1016/j.euo.2021.11.005 10.1016/j.euo.2021.11.005 - DOI - PMC - PubMed
-
- Tagawa ST, Balar AV, Petrylak DP, et al. . TROPHY-U-01: A Phase II Open-Label Study of Sacituzumab Govitecan in Patients With Metastatic Urothelial Carcinoma Progressing After Platinum-Based Chemotherapy and Checkpoint Inhibitors. J Clin Oncol Off J Am Soc Clin Oncol. 2021;39:2474-2485. https://doi.org/10.1200/JCO.20.03489 10.1200/JCO.20.03489 - DOI - PMC - PubMed
-
- Klümper N, Ralser DJ, Ellinger J, et al. . Membranous NECTIN-4 Expression Frequently Decreases during Metastatic Spread of Urothelial Carcinoma and Is Associated with Enfortumab Vedotin Resistance. Clin Cancer Res Off J Am Assoc Cancer Res. 2023;29:1496-1505. https://doi.org/10.1158/1078-0432.CCR-22-1764 10.1158/1078-0432.CCR-22-1764 - DOI - PMC - PubMed
-
- O’Donnell PH, Milowsky MI, Petrylak DP, et al. . Enfortumab Vedotin With or Without Pembrolizumab in Cisplatin-Ineligible Patients With Previously Untreated Locally Advanced or Metastatic Urothelial Cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2023;JCO2202887. https://doi.org/10.1200/JCO.22.02887 10.1200/JCO.22.02887 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

