Comparative Risk of Injury with Concurrent Use of Opioids and Skeletal Muscle Relaxants
- PMID: 38482733
- PMCID: PMC11180590
- DOI: 10.1002/cpt.3248
Comparative Risk of Injury with Concurrent Use of Opioids and Skeletal Muscle Relaxants
Abstract
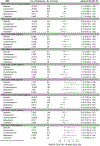
Concurrent use of skeletal muscle relaxants (SMRs) and opioids has been linked to an increased risk of injury. However, it remains unclear whether the injury risks differ by specific SMR when combined with opioids. We conducted nine retrospective cohort studies within a US Medicaid population. Each cohort consisted exclusively of person-time exposed to both an SMR and one of the three most dispensed opioids-hydrocodone, oxycodone, and tramadol. Opioid users were further divided into three cohorts based on the initiation order of SMRs and opioids-synchronically triggered, opioid-triggered, and SMR-triggered. Within each cohort, we used Cox proportional hazard models to compare the injury rates for different SMRs compared to methocarbamol, adjusting for covariates. We identified 349,543, 139,458, and 218,967 concurrent users of SMRs with hydrocodone, oxycodone, and tramadol, respectively. In the oxycodone-SMR-triggered cohort, the adjusted hazard ratios (HRs) were 1.86 (95% CI, 1.23-2.82) for carisoprodol and 1.73 (1.09-2.73) for tizanidine. In the tramadol-synchronically triggered cohort, the adjusted HRs were 0.69 (0.49-0.97) for metaxalone and 0.62 (0.42-0.90) for tizanidine. In the tramadol-SMR-triggered cohort, the adjusted HRs were 1.51 (1.01-2.26) for baclofen and 1.48 (1.03-2.11) for cyclobenzaprine. All other HRs were statistically nonsignificant. In conclusion, the relative injury rate associated with different SMRs used concurrently with the three most dispensed opioids appears to vary depending on the specific opioid and the order of combination initiation. If confirmed by future studies, clinicians should consider the varying injury rates when prescribing SMRs to individuals using hydrocodone, oxycodone, and tramadol.
© 2024 The Authors. Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
Dr.Chen is currently an employee of the U.S. Food and Drug Administration. The research was conducted while she was a postdocoral researcher at the University of Pennsylvania. Dr. Leonard is an Executive Committee Member of and Dr. Hennessy directs the University of Pennsylvania’s Center for Real-World Effectiveness and Safety of Therapeutics. The Center receives funds from Pfizer and Sanofi to support pharmacoepidemiology education. Dr. Leonard recently received honoraria from the American College of Clinical Pharmacy Foundation, the Scientific and Data Coordinating Center for the NIDDK-funded Chronic Renal Insufficiency Cohort Study, and the Consortium for Medical Marijuana Clinical Outcomes Research. Dr. Leonard is a Special Government Employee of the United States (US) Food and Drug Administration and recently consulted for their Reagan-Udall Foundation. Dr. Leonard receives travel support from John Wiley & Sons. Dr. Leonard’s spouse is an employee of Merck; neither Dr. Leonard nor his spouse owns stock in the company. Dr. Hennessy has consulted for Covis Pharma GmbH, the Medullary Thyroid Cancer Consortium (Novo Nordisk Inc, AstraZeneca Pharmaceuticals LP, and Eli Lilly Company), Urvant Sciences and i2o Therapeutics. Dr.Bilker is a paid consultant for Genentech for unrelated medications. Dr.Dublin was a co-Investigator on Kaiser Permanente Washington Health Research Institute projects funded by Syneos Health, who is representing a consortium of pharmaceutical companies carrying out studies mandated by the US Food and Drug Administration regarding the safety of extended-release opioids. All other authors declared no competing interests for this work.
Figures

References
-
- Li Y et al. Utilization Patterns of Skeletal Muscle Relaxants Among Commercially Insured Adults in the United States from 2006 to 2018. Pain Med 22, 2153–2161 (2021). - PubMed
-
- Musich S, Wang SS, Slindee L, Kraemer S & Yeh CS Characteristics associated with transition from opioid initiation to chronic opioid use among opioid-naïve older adults. Geriatr Nurs 40, 190–196 (2019). - PubMed
-
- Larochelle MR, Zhang F, Ross-Degnan D & Wharam JF Trends in opioid prescribing and co-prescribing of sedative hypnotics for acute and chronic musculoskeletal pain: 2001–2010. Pharmacoepidemiol Drug Saf 24, 885–892 (2015). - PubMed
-
- FDA Drug Safety Communication: FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines; requires its strongest warning. FDA (2019).at <https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-c...>
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

