C9orf72 polyPR directly binds to various nuclear transport components
- PMID: 38483313
- PMCID: PMC10939497
- DOI: 10.7554/eLife.89694
C9orf72 polyPR directly binds to various nuclear transport components
Abstract
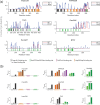
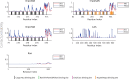
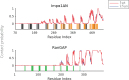
The disruption of nucleocytoplasmic transport (NCT) is an important mechanism in neurodegenerative diseases. In the case of C9orf72-ALS, trafficking of macromolecules through the nuclear pore complex (NPC) might get frustrated by the binding of C9orf72-translated arginine-containing dipeptide repeat proteins (R-DPRs) to the Kapβ family of nuclear transport receptors. Besides Kapβs, several other types of transport components have been linked to NCT impairments in R-DPR-expressed cells, but the molecular origin of these observations has not been clarified. Here, we adopt a coarse-grained molecular dynamics model at amino acid resolution to study the direct interaction between polyPR, the most toxic DPR, and various nuclear transport components to elucidate the binding mechanisms and provide a complete picture of potential polyPR-mediated NCT defects. We found polyPR to directly bind to several isoforms of the Impα family, CAS (the specific exporter of Impα) and RanGAP. We observe no binding between polyPR and Ran. Longer polyPRs at lower salt concentrations also make contact with RanGEF and NTF2. Analyzing the polyPR contact sites on the transport components reveals that polyPR potentially interferes with RanGTP/RanGDP binding, with nuclear localization signal (NLS)-containing cargoes (cargo-NLS) binding to Impα, with cargo-NLS release from Impα, and with Impα export from the nucleus. The abundance of polyPR-binding sites on multiple transport components combined with the inherent polyPR length dependence makes direct polyPR interference of NCT a potential mechanistic pathway of C9orf72 toxicity.
Keywords: ALS/FTD; C9orf72; importins/exportins; molecular dynamics; neuroscience; none; nuclear pore complex; nucleocytoplasmic transport; physics of living systems.
© 2023, Jafarinia et al.
Conflict of interest statement
HJ, Ev, PO No competing interests declared
Figures




Update of
- doi: 10.1101/2023.06.22.546068
- doi: 10.7554/eLife.89694.1
- doi: 10.7554/eLife.89694.2
Similar articles
-
C9orf72 polyPR interaction with the nuclear pore complex.Biophys J. 2024 Oct 15;123(20):3533-3539. doi: 10.1016/j.bpj.2024.08.024. Epub 2024 Aug 30. Biophys J. 2024. PMID: 39205388
-
Molecular basis of C9orf72 poly-PR interference with the β-karyopherin family of nuclear transport receptors.Sci Rep. 2022 Dec 9;12(1):21324. doi: 10.1038/s41598-022-25732-y. Sci Rep. 2022. PMID: 36494425 Free PMC article.
-
C9orf72 proline-arginine dipeptide repeats disrupt the proteasome and perturb proteolytic activities.J Neuropathol Exp Neurol. 2023 Oct 20;82(11):901-910. doi: 10.1093/jnen/nlad078. J Neuropathol Exp Neurol. 2023. PMID: 37791472 Free PMC article.
-
On the asymmetric partitioning of nucleocytoplasmic transport - recent insights and open questions.J Cell Sci. 2021 Apr 1;134(7):jcs240382. doi: 10.1242/jcs.240382. Epub 2021 Apr 13. J Cell Sci. 2021. PMID: 33912945 Review.
-
C9orf72 ALS-FTD: recent evidence for dysregulation of the autophagy-lysosome pathway at multiple levels.Autophagy. 2021 Nov;17(11):3306-3322. doi: 10.1080/15548627.2021.1872189. Epub 2021 Feb 26. Autophagy. 2021. PMID: 33632058 Free PMC article. Review.
Cited by
-
Targeting Gene C9orf72 Pathogenesis for Amyotrophic Lateral Sclerosis.Int J Mol Sci. 2025 Apr 30;26(9):4276. doi: 10.3390/ijms26094276. Int J Mol Sci. 2025. PMID: 40362512 Free PMC article. Review.
-
Protein folding and quality control during nuclear transport.Curr Opin Cell Biol. 2024 Oct;90:102407. doi: 10.1016/j.ceb.2024.102407. Epub 2024 Aug 13. Curr Opin Cell Biol. 2024. PMID: 39142062 Free PMC article. Review.
-
Blueprint of Collapse: Precision Biomarkers, Molecular Cascades, and the Engineered Decline of Fast-Progressing ALS.Int J Mol Sci. 2025 Aug 21;26(16):8072. doi: 10.3390/ijms26168072. Int J Mol Sci. 2025. PMID: 40869392 Free PMC article. Review.
-
RNA gain-of-function mechanisms in short tandem repeat diseases.RNA. 2025 Feb 19;31(3):349-358. doi: 10.1261/rna.080277.124. RNA. 2025. PMID: 39725460 Free PMC article. Review.
-
C9orf72 polyPR interaction with the nuclear pore complex.Biophys J. 2024 Oct 15;123(20):3533-3539. doi: 10.1016/j.bpj.2024.08.024. Epub 2024 Aug 30. Biophys J. 2024. PMID: 39205388
References
-
- Boeynaems S, Bogaert E, Michiels E, Gijselinck I, Sieben A, Jovičić A, De Baets G, Scheveneels W, Steyaert J, Cuijt I, Verstrepen KJ, Callaerts P, Rousseau F, Schymkowitz J, Cruts M, Van Broeckhoven C, Van Damme P, Gitler AD, Robberecht W, Van Den Bosch L. Drosophila screen connects nuclear transport genes to DPR pathology in c9ALS/FTD. Scientific Reports. 2016a;6:20877. doi: 10.1038/srep20877. - DOI - PMC - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Miscellaneous

