Outpatient COVID-19 convalescent plasma recipient antibody thresholds correlated to reduced hospitalizations within a randomized trial
- PMID: 38483534
- PMCID: PMC11141865
- DOI: 10.1172/jci.insight.178460
Outpatient COVID-19 convalescent plasma recipient antibody thresholds correlated to reduced hospitalizations within a randomized trial
Abstract
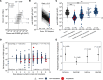
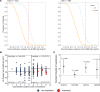
BACKGROUNDCOVID-19 convalescent plasma (CCP) virus-specific antibody levels that translate into recipient posttransfusion antibody levels sufficient to prevent disease progression are not defined.METHODSThis secondary analysis correlated donor and recipient antibody levels to hospitalization risk among unvaccinated, seronegative CCP recipients within the outpatient, double-blind, randomized clinical trial that compared CCP to control plasma. The majority of COVID-19 CCP arm hospitalizations (15/17, 88%) occurred in this unvaccinated, seronegative subgroup. A functional cutoff to delineate recipient high versus low posttransfusion antibody levels was established by 2 methods: (i) analyzing virus neutralization-equivalent anti-Spike receptor-binding domain immunoglobulin G (anti-S-RBD IgG) responses in donors or (ii) receiver operating characteristic (ROC) curve analysis.RESULTSSARS-CoV-2 anti-S-RBD IgG antibody was volume diluted 21.3-fold into posttransfusion seronegative recipients from matched donor units. Virus-specific antibody delivered was approximately 1.2 mg. The high-antibody recipients transfused early (symptom onset within 5 days) had no hospitalizations. A CCP-recipient analysis for antibody thresholds correlated to reduced hospitalizations found a statistical significant association between early transfusion and high antibodies versus all other CCP recipients (or control plasma), with antibody cutoffs established by both methods-donor-based virus neutralization cutoffs in posttransfusion recipients (0/85 [0%] versus 15/276 [5.6%]; P = 0.03) or ROC-based cutoff (0/94 [0%] versus 15/267 [5.4%]; P = 0.01).CONCLUSIONIn unvaccinated, seronegative CCP recipients, early transfusion of plasma units in the upper 30% of study donors' antibody levels reduced outpatient hospitalizations. High antibody level plasma units, given early, should be reserved for therapeutic use.TRIAL REGISTRATIONClinicalTrials.gov NCT04373460.FUNDINGDepartment of Defense (W911QY2090012); Defense Health Agency; Bloomberg Philanthropies; the State of Maryland; NIH (3R01AI152078-01S1, U24TR001609-S3, 1K23HL151826NIH); the Mental Wellness Foundation; the Moriah Fund; Octapharma; the Healthnetwork Foundation; the Shear Family Foundation; the NorthShore Research Institute; and the Rice Foundation.
Keywords: COVID-19; Immunoglobulins; Immunotherapy.
Figures

Update of
-
Outpatient COVID-19 convalescent plasma recipient antibody thresholds correlated to reduced hospitalizations within a randomized trial.medRxiv [Preprint]. 2023 Dec 15:2023.04.13.23288353. doi: 10.1101/2023.04.13.23288353. medRxiv. 2023. Update in: JCI Insight. 2024 Mar 14;9(8):e178460. doi: 10.1172/jci.insight.178460. PMID: 37131659 Free PMC article. Updated. Preprint.
References
-
- Zhang S, et al. High-throughput neutralization and serology assays reveal correlated but highly variable humoral immune responses in a large population of individuals infected with SARS-CoV-2 in the US between March and August 2020. mBio. 2023;14(2):e0352322. doi: 10.1128/mbio.03523-22. - DOI - PMC - PubMed

