Antivirals to prepare for surges in influenza cases: an economic evaluation of baloxavir marboxil for the Netherlands
- PMID: 38483666
- PMCID: PMC11512865
- DOI: 10.1007/s10198-024-01683-1
Antivirals to prepare for surges in influenza cases: an economic evaluation of baloxavir marboxil for the Netherlands
Abstract
Objectives: We perform a cost-effectiveness analysis (CEA) and budget impact analysis (BIA) of baloxavir marboxil compared to current care in the Netherlands for patients at risk of influenza-related complications, including patients with comorbidities and the elderly.
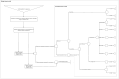
Methods: In the CEA, a decision tree model was developed to assess the cost-effectiveness of baloxavir marboxil for a cohort of 52-year-olds from a societal perspective. A lifetime horizon was taken by incorporating the quality-adjusted life expectancy. The BIA included different epidemiological scenarios, estimating different plausible epidemiological scenarios for seasonal influenza considering the whole Dutch population with an increased risk of influenza complications.
Results: The base-case ICER was estimated to be €8,300 per QALY. At the willingness-to-pay threshold of €20,000 per QALY, the probability of being cost effective was 58%. The base-case expected budget impact was €5.7 million on average per year, ranging from €1.5 million to €10.5 million based on the severity of the influenza epidemic and vaccine effectiveness.
Conclusion: In the Netherlands, baloxavir is a cost-effective treatment option for seasonal influenza, with a base-case ICER of €8,300 per QALY for the population aged 60 years and over and patients at high risk of influenza-related complications. For a large part, this ICER is driven by the reduction of the illness duration of influenza and productivity gains in the working population.
Keywords: Antiviral; Baloxavir; Budget impact; Cost effectiveness; Seasonal influenza.
© 2024. The Author(s).
Conflict of interest statement
Prof. Dr. Maarten J. Postma and Prof. Dr. Cornelis Boersma received grants and honoraria from various pharmaceutical companies, inclusive those developing, producing, and marketing influenza antivirals and vaccines. Dr. Simon van der Pol has no relevant financial or non-financial interests to disclose.
Figures
References
-
- Teirlinck, A.C., de Gier, B., Meijer, A., Donker, G., de Lange, M., Koppeschaar, C., van der Hoek, W., Kretzschmar, M.E., McDonald, S.A.: The incidence of symptomatic infection with influenza virus in the Netherlands 2011/2012 through 2016/2017, estimated using Bayesian evidence synthesis. Epidemiol. Infect. (2019). 10.1017/S095026881800273X - PMC - PubMed
-
- Gezondheidsraad: Vaccinatie risicogroepen op grond van leeftijd en medische indicatie. Den Haag (2021). https://www.gezondheidsraad.nl/binaries/gezondheidsraad/documenten/advie...
-
- LCI richtlijnen. Influenza. https://lci.rivm.nl/richtlijnen/influenza. Accessed 17 Dec 2021
-
- World Health Organization WHO FLUMART OUTPUTS. https://apps.who.int/flumart/Default?ReportNo=16
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical