Structural insights reveal interplay between LAG-3 homodimerization, ligand binding, and function
- PMID: 38483996
- PMCID: PMC10962948
- DOI: 10.1073/pnas.2310866121
Structural insights reveal interplay between LAG-3 homodimerization, ligand binding, and function
Abstract
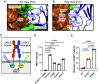
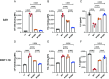
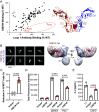
Lymphocyte activation gene-3 (LAG-3) is an inhibitory receptor expressed on activated T cells and an emerging immunotherapy target. Domain 1 (D1) of LAG-3, which has been purported to directly interact with major histocompatibility complex class II (MHCII) and fibrinogen-like protein 1 (FGL1), has been the major focus for the development of therapeutic antibodies that inhibit LAG-3 receptor-ligand interactions and restore T cell function. Here, we present a high-resolution structure of glycosylated mouse LAG-3 ectodomain, identifying that cis-homodimerization, mediated through a network of hydrophobic residues within domain 2 (D2), is critically required for LAG-3 function. Additionally, we found a previously unidentified key protein-glycan interaction in the dimer interface that affects the spatial orientation of the neighboring D1 domain. Mutation of LAG-3 D2 residues reduced dimer formation, dramatically abolished LAG-3 binding to both MHCII and FGL1 ligands, and consequentially inhibited the role of LAG-3 in suppressing T cell responses. Intriguingly, we showed that antibodies directed against D1, D2, and D3 domains are all capable of blocking LAG-3 dimer formation and MHCII and FGL-1 ligand binding, suggesting a potential allosteric model of LAG-3 function tightly regulated by dimerization. Furthermore, our work reveals unique epitopes, in addition to D1, that can be targeted for immunotherapy of cancer and other human diseases.
Keywords: LAG-3; cancer immunotherapy; dimerization; immune checkpoint; structural biology.
Conflict of interest statement
Competing interests statement:Authors are inventors on intellectual property related to this work that is owned by Stanford University and New York University. J.W. is on the Scientific Advisory Board of Rootpath Genomics and is a consultant for BMS (Relatlimab Advisory Council) and Beijing Hanmi Pharmaceutical Co., LTD. J.R.C. is a cofounder and equity holder of Combangio, Inc. (now Kala Bio), xCella Biosciences (now OmniAb), Charged Biotherapeutics, TwoStep Therapeutics, and Red Tree Venture Capital; has financial interests in Aravive, Inc.; is a member of the Board of Directors of OmniAb, Revel Pharmaceuticals, Excellergy Therapeutics, Rondo Therapeutics, Tachyon Therapeutics, and Biograph 55; and is a Board Observer at Acrigen Biosciences. The other authors have no competing interests.
Figures



References
-
- Workman C. J., Dugger K. J., Vignali D. A. A., Cutting edge: Molecular analysis of the negative regulatory function of lymphocyte activation gene-31. J. Immunol. 169, 5392–5395 (2002). - PubMed
-
- Richter K., Agnellini P., Oxenius A., On the role of the inhibitory receptor LAG-3 in acute and chronic LCMV infection. Int. Immunol. 22, 13–23 (2010). - PubMed
MeSH terms
Substances
Grants and funding
- R01CA269898/GF/NIH HHS/United States
- R21AI163924-02/GF/NIH HHS/United States
- R37 CA273333/CA/NCI NIH HHS/United States
- S10 OD021727/OD/NIH HHS/United States
- R37CA273333-01/GF/NIH HHS/United States
- R01 CA269898/CA/NCI NIH HHS/United States
- P30 CA016087/CA/NCI NIH HHS/United States
- R21 AI163924/AI/NIAID NIH HHS/United States
- T32 AI007290/AI/NIAID NIH HHS/United States
- P30 GM133894/GM/NIGMS NIH HHS/United States
- P50 CA225450/CA/NCI NIH HHS/United States
- T32 AR069515/AR/NIAMS NIH HHS/United States
- T32 GM136568/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

