Economic evaluation: immunoglobulin vs prophylactic antibiotics in hypogammaglobulinemia and hematological malignancies
- PMID: 38484199
- PMCID: PMC11116992
- DOI: 10.1182/bloodadvances.2023012047
Economic evaluation: immunoglobulin vs prophylactic antibiotics in hypogammaglobulinemia and hematological malignancies
Abstract
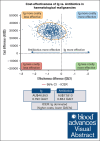
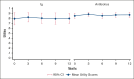
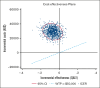
Patients with hematological malignancies are at high risk of developing hypogammaglobulinemia (HGG) and infections. Immunoglobulin (Ig) is one recommended option to prevent these infections, but it is expensive, and its cost-effectiveness compared with other prevention strategies remains unknown. We conducted a trial-based economic evaluation from the Australian health care system perspective to estimate the 12-month cost-effectiveness of prophylactic Ig vs prophylactic antibiotics in 63 adults with HGG and hematological malignancies participating in the RATIONAL feasibility trial. Two analyses were conducted: (1) cost-utility analysis to assess the incremental cost per quality-adjusted life year (QALY) gained; and (2) cost-effectiveness analysis to assess the incremental cost per serious infection prevented (grade ≥3) and per any infection (any grade) prevented. Over 12 months, the total cost per patient was significantly higher in the Ig group than in the antibiotic group (mean difference, AU$29 140; P < .001). Most patients received IVIg, which was the main cost driver; only 2 patients in the intervention arm received subcutaneous Ig. There were nonsignificant differences in health outcomes. Results showed Ig was more costly than antibiotics and associated with fewer QALYs. The incremental cost-effectiveness ratio of Ig vs antibiotics was AU$111 262 per serious infection prevented, but Ig was more costly and associated with more infections when all infections were included. On average and for this patient population, Ig prophylaxis may not be cost-effective compared with prophylactic antibiotics. Further research is needed to confirm these findings in a larger population and considering longer-term outcomes. The trial was registered at the Australian and New Zealand Clinical Trials Registry as #ACTRN12616001723471.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: R.W. reports advisory board and speaker fees from Janssen and AbbVie; and research funding to institution from Janssen, BioOra, and Wellington Zhaotai Therapies Limited. J.R. is an equity holder in the publicly traded company Novartis and Alcon. J.T. reports research funding to institution from Janssen, BeiGene, Roche, Bristol Myers Squibb, and Cellectar Biosciences. A.J. participated in advisory boards and received honoraria from Roche, Link, MSD, BeiGene, Sanofi, EUSA Pharma, and Novartis; and provided medical education for Takeda. E.M.W. and Z.K.M. received grant funding from CSL Behring not related to this study; and research funding to institution from AbbVie, Amgen, AstraZeneca, BeiGene, Celgene, Janssen, New Zealand Blood Service, Novartis, Sanofi, and Takeda. The remaining authors declare no competing financial interests.
Figures
References
-
- Benbrahim O, Viallard JF, Choquet S, et al. A French observational study describing the use of human polyvalent immunoglobulins in hematological malignancy-associated secondary immunodeficiency. Eur J Haematol. 2018;101(1):48–56. - PubMed
-
- Friman V, Winqvist O, Blimark C, Langerbeins P, Chapel H, Dhalla F. Secondary immunodeficiency in lymphoproliferative malignancies. Hematol Oncol. 2016;34(3):121–132. - PubMed
-
- European Medicines Agency Guideline on Core SmPC for Human Normal Immunoglobulin for Intravenous Administration (IVIg) 2018. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-co...
-
- UK Department of Health Clinical Guidelines for Immunoglobulin Use, Update to 2nd Edition. 2011. https://assets.publishing.service.gov.uk/government/uploads/system/uploa...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

