Membrane Proteome-Wide Screening of Autoantibodies in CIDP Using Human Cell Microarray Technology
- PMID: 38484217
- PMCID: PMC11078148
- DOI: 10.1212/NXI.0000000000200216
Membrane Proteome-Wide Screening of Autoantibodies in CIDP Using Human Cell Microarray Technology
Abstract
Background and objectives: Autoantibody discovery in complex autoimmune diseases is challenging. Diverse successful antigen identification strategies are available, but, so far, have often been unsuccessful, especially in the discovery of protein antigens in which conformational and post-translational modification are critical. Our study assesses the utility of a human membrane and secreted protein microarray technology to detect autoantibodies in chronic inflammatory demyelinating polyradiculoneuropathy (CIDP).
Methods: A cell microarray consisting of human embryonic kidney-293 cells expressing >5,000 human proteins was used. First, a validation step was performed with 4 serum samples from patients with autoimmune nodopathy (AN) to assess the ability of this technology to detect circulating known autoantibodies. The ability of the cell microarray technology to discover novel IgG autoantibodies was assessed incubating the array with 8 CIDP serum samples. Identified autoantibodies were subsequently validated using cell-based assays (CBAs), ELISA, and/or tissue immunohistochemistry and analyzed in a cohort of CIDP and AN (n = 96) and control (n = 100) samples.
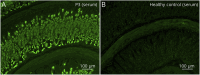
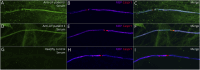
Results: Serum anti-contactin-1 and anti-neurofascin-155 were detected by the human cell microarray technology. Nine potentially relevant antigens were found in patients with CIDP without other detectable antibodies; confirmation was possible in six of them: ephrin type-A receptor 7 (EPHA7); potassium-transporting ATPase alpha chain 1 and subunit beta (ATP4A/4B); leukemia-inhibitory factor (LIF); and interferon lambda 1, 2, and 3 (IFNL1, IFNL2, IFNL3). Anti-ATP4A/4B and anti-EPHA7 antibodies were detected in patients and controls and considered unrelated to CIDP. Both anti-LIF and anti-IFNL antibodies were found in the same 2 patients and were not detected in any control. Both patients showed the same staining pattern against myelinating fibers of peripheral nerve tissue and of myelinating neuron-Schwann cell cocultures. Clinically relevant correlations could not be established for anti-LIF and anti-IFNL3 antibodies.
Discussion: Our work demonstrates the utility of human cell microarray technology to detect known and discover unknown autoantibodies in human serum samples. Despite potential CIDP-associated autoantibodies (anti-LIF and anti-IFNL3) being identified, their clinical and pathogenic relevance needs to be elucidated in bigger cohorts.
Conflict of interest statement
M. Caballero-Ávila was supported by a personal Rio Hortega grant CM21/00101. L. Martín-Aguilar was supported by a personal Juan Rodés grant JR21/00060. E. Pascual-Goñi was supported by a personal grant from the GBS-CIDP foundation. A. Carbayo was supported by a personal Rio Hortega grant CM21/00057. L. Querol received research grants from Instituto de Salud Carlos III-Ministry of Economy and Innovation (Spain), CIBERER, Fundació La Marató, GBS-CIDP Foundation International, UCB, and Grifols; received speaker or expert testimony honoraria from CSL Behring, Novartis, Sanofi-Genzyme, Merck, Annexon, Alnylam, Biogen, Janssen, Lundbeck, ArgenX, UCB, LFB, Octapharma and Roche; serves at Clinical Trial Steering Committee for Sanofi-Genzyme and Roche and is Principal Investigator for UCB's CIDP01 trial. L. Querol was supported by a personal clinical intensification grant INT20/00080. J. Freeth and J. Soden were former employees for Retrogenix, a company that was acquired by Charles River. S. Dawson is a Charles River employee. All other authors report no disclosures relevant to the manuscript. Go to
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
