Metabolic priming by multiple enzyme systems supports glycolysis, HIF1α stabilisation, and human cancer cell survival in early hypoxia
- PMID: 38485816
- PMCID: PMC11021510
- DOI: 10.1038/s44318-024-00065-w
Metabolic priming by multiple enzyme systems supports glycolysis, HIF1α stabilisation, and human cancer cell survival in early hypoxia
Abstract
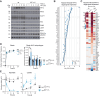
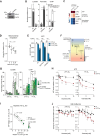
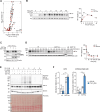
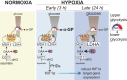
Adaptation to chronic hypoxia occurs through changes in protein expression, which are controlled by hypoxia-inducible factor 1α (HIF1α) and are necessary for cancer cell survival. However, the mechanisms that enable cancer cells to adapt in early hypoxia, before the HIF1α-mediated transcription programme is fully established, remain poorly understood. Here we show in human breast cancer cells, that within 3 h of hypoxia exposure, glycolytic flux increases in a HIF1α-independent manner but is limited by NAD+ availability. Glycolytic ATP maintenance and cell survival in early hypoxia rely on reserve lactate dehydrogenase A capacity as well as the activity of glutamate-oxoglutarate transaminase 1 (GOT1), an enzyme that fuels malate dehydrogenase 1 (MDH1)-derived NAD+. In addition, GOT1 maintains low α-ketoglutarate levels, thereby limiting prolyl hydroxylase activity to promote HIF1α stabilisation in early hypoxia and enable robust HIF1α target gene expression in later hypoxia. Our findings reveal that, in normoxia, multiple enzyme systems maintain cells in a primed state ready to support increased glycolysis and HIF1α stabilisation upon oxygen limitation, until other adaptive processes that require more time are fully established.
Keywords: Glycolysis; HIF1α; Hypoxia; Metabolism; α-Ketoglutarate.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Abboud MI, McAllister TE, Leung IKH, Chowdhury R, Jorgensen C, Domene C, Mecinovic J, Lippl K, Hancock RL, Hopkinson RJ, et al. 2-Oxoglutarate regulates binding of hydroxylated hypoxia-inducible factor to prolyl hydroxylase domain 2. Chem Commun. 2018;54:3130–3133. doi: 10.1039/C8CC00387D. - DOI - PMC - PubMed
-
- Altea-Manzano P, Vandekeere A, Edwards-Hicks J, Roldan M, Abraham E, Lleshi X, Guerrieri AN, Berardi D, Wills J, Junior JM, et al. Reversal of mitochondrial malate dehydrogenase 2 enables anaplerosis via redox rescue in respiration-deficient cells. Mol Cell. 2022;82:4537–4547.e4537. doi: 10.1016/j.molcel.2022.10.005. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

