MCT4-driven CAF-mediated metabolic reprogramming in breast cancer microenvironment is a vulnerability targetable by miR-425-5p
- PMID: 38485929
- PMCID: PMC10940713
- DOI: 10.1038/s41420-024-01910-x
MCT4-driven CAF-mediated metabolic reprogramming in breast cancer microenvironment is a vulnerability targetable by miR-425-5p
Abstract
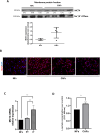
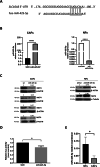
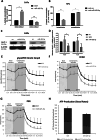
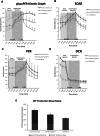
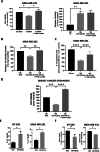
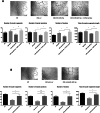
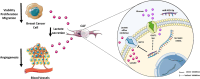
Multiple oncogenic alterations contribute to breast cancer development. Metabolic reprogramming, deeply contributing to tumor microenvironment (TME) education, is now widely recognized as a hallmark of cancer. The reverse Warburg effect induces cancer-associated fibroblasts (CAFs) to produce and secrete L-lactate, enhancing malignant characteristics such as neoangiogenesis, metastatic dissemination, and treatment resistance. Monocarboxylate transporter (MCT) 4 is involved in lactate efflux from CAFs into stromal and epithelial cells. Here, we first assess the expression of miR-425-5p and its target MCT4 in breast cancer CAFs and normal fibroblasts. We analyzed the metabolic changes induced by miR-425-5p in CAFs and its role in the education of breast cancer epithelial cells. We show that miR-425-5p-induced MCT4 knockdown decreased lactate extrusion from CAFs and its availability in the TME. miR-425-5p overexpression induced profound metabolic transformation in CAFs, ultimately influencing breast cancer metabolism. Furthermore, miR-425-5p impaired the capacity of CAFs to sustain vessel formation and breast cancer cell migration, viability, and proliferation. These findings emphasize the key role of miR-425-5p in breast cancer metabolism and aggressiveness, and its possible importance for breast cancer therapy and monitoring.
© 2024. The Author(s).
Conflict of interest statement
All authors have read and approved the manuscript, its contents, and its submission and disclose no potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources

