In vivo affinity maturation of mouse B cells reprogrammed to express human antibodies
- PMID: 38486104
- PMCID: PMC12090756
- DOI: 10.1038/s41551-024-01179-6
In vivo affinity maturation of mouse B cells reprogrammed to express human antibodies
Abstract
Mice adoptively transferred with mouse B cells edited via CRISPR to express human antibody variable chains could help evaluate candidate vaccines and develop better antibody therapies. However, current editing strategies disrupt the heavy-chain locus, resulting in inefficient somatic hypermutation without functional affinity maturation. Here we show that these key B-cell functions can be preserved by directly and simultaneously replacing recombined mouse heavy and kappa chains with those of human antibodies, using a single Cas12a-mediated cut at each locus and 5' homology arms complementary to distal V segments. Cells edited in this way to express the human immunodeficiency virus type 1 (HIV-1) broadly neutralizing antibody 10-1074 or VRC26.25-y robustly hypermutated and generated potent neutralizing plasma in vaccinated mice. The 10-1074 variants isolated from the mice neutralized a global panel of HIV-1 isolates more efficiently than wild-type 10-1074 while maintaining its low polyreactivity and long half-life. We also used the approach to improve the potency of anti-SARS-CoV-2 antibodies against recent Omicron strains. In vivo affinity maturation of B cells edited at their native loci may facilitate the development of broad, potent and bioavailable antibodies.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Figures
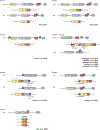
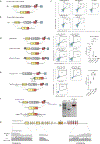


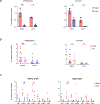


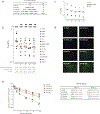


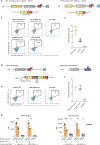
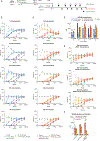





Update of
-
In vivo affinity maturation of murine B cells reprogrammed to express human antibodies.bioRxiv [Preprint]. 2023 Oct 23:2023.10.20.563154. doi: 10.1101/2023.10.20.563154. bioRxiv. 2023. Update in: Nat Biomed Eng. 2024 Apr;8(4):361-379. doi: 10.1038/s41551-024-01179-6. PMID: 37961481 Free PMC article. Updated. Preprint.
Similar articles
-
In vivo affinity maturation of murine B cells reprogrammed to express human antibodies.bioRxiv [Preprint]. 2023 Oct 23:2023.10.20.563154. doi: 10.1101/2023.10.20.563154. bioRxiv. 2023. Update in: Nat Biomed Eng. 2024 Apr;8(4):361-379. doi: 10.1038/s41551-024-01179-6. PMID: 37961481 Free PMC article. Updated. Preprint.
-
In vitro affinity maturation of broader and more-potent variants of the HIV-1-neutralizing antibody CAP256-VRC26.25.Proc Natl Acad Sci U S A. 2021 Jul 20;118(29):e2106203118. doi: 10.1073/pnas.2106203118. Proc Natl Acad Sci U S A. 2021. PMID: 34261793 Free PMC article.
-
Novel Monoclonal Antibodies and Recombined Antibodies Against Variant SARS-CoV-2.Front Immunol. 2021 Aug 30;12:715464. doi: 10.3389/fimmu.2021.715464. eCollection 2021. Front Immunol. 2021. PMID: 34539645 Free PMC article.
-
Comprehensive Overview of Broadly Neutralizing Antibodies against SARS-CoV-2 Variants.Viruses. 2024 Jun 1;16(6):900. doi: 10.3390/v16060900. Viruses. 2024. PMID: 38932192 Free PMC article. Review.
-
Unravelling humoral immunity in SARS-CoV-2: Insights from infection and vaccination.Hum Antibodies. 2024;32(3):85-106. doi: 10.3233/HAB-230017. Hum Antibodies. 2024. PMID: 38758995 Review.
Cited by
-
In vivo affinity maturation of the HIV-1 Env-binding domain of CD4.bioRxiv [Preprint]. 2024 Feb 5:2024.02.03.578630. doi: 10.1101/2024.02.03.578630. bioRxiv. 2024. Update in: Nat Biomed Eng. 2024 Dec;8(12):1715-1729. doi: 10.1038/s41551-024-01289-1. PMID: 38370774 Free PMC article. Updated. Preprint.
-
Interventions during Early Infection: Opening a Window for an HIV Cure?Viruses. 2024 Oct 9;16(10):1588. doi: 10.3390/v16101588. Viruses. 2024. PMID: 39459922 Free PMC article. Review.
-
The HIV-1 envelope glycoprotein: structure, function and interactions with neutralizing antibodies.Nat Rev Microbiol. 2025 Jul 23. doi: 10.1038/s41579-025-01206-6. Online ahead of print. Nat Rev Microbiol. 2025. PMID: 40702326 Review.
-
In vivo affinity maturation of the HIV-1 Env-binding domain of CD4.Res Sq [Preprint]. 2024 Feb 9:rs.3.rs-3922904. doi: 10.21203/rs.3.rs-3922904/v1. Res Sq. 2024. Update in: Nat Biomed Eng. 2024 Dec;8(12):1715-1729. doi: 10.1038/s41551-024-01289-1. PMID: 38405717 Free PMC article. Updated. Preprint.
-
Mouse B cells engineered to express an anti-HPV antibody elicit anti-tumor T cell responses.Front Immunol. 2025 Jul 18;16:1613879. doi: 10.3389/fimmu.2025.1613879. eCollection 2025. Front Immunol. 2025. PMID: 40755763 Free PMC article.
References
-
- Hoogenboom HR Selecting and screening recombinant antibody libraries. Nature biotechnology. 23, 1105–1116 (2005). - PubMed
-
- Shih TA, Meffre E, Roederer M, & Nussenzweig MC Role of BCR affinity in T cell–dependent antibody responses in vivo. Nature immunology. 3, 570–575 (2002). - PubMed
-
- Wardemann H. et al. Predominant autoantibody production by early human B cell precursors. Science. 301, 1374–1377 (2003). - PubMed
-
- Jarasch A. et al. Developability assessment during the selection of novel therapeutic antibodies. Journal of pharmaceutical sciences. 104, 1885–1898 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

