Analysis of drug-induced interstitial lung disease caused by herbal medicine using the Japanese Adverse Drug Event Report database
- PMID: 38486172
- PMCID: PMC10938654
- DOI: 10.1186/s12906-024-04428-y
Analysis of drug-induced interstitial lung disease caused by herbal medicine using the Japanese Adverse Drug Event Report database
Abstract
Background: Drug-induced interstitial lung disease (DIILD) is a severe adverse event leading to morbidity and mortality. This study evaluated the adverse event indicators of DIILD and time-to-onset profiles following the daily intake of herbal drugs (Scutellariae radix ["ogon" in Japanese], Bupleuri radix ["saiko" in Japanese], and Pinelliae tuber ["hange" in Japanese]) using the Japanese Adverse Drug Event Report database. DIILD was defined in accordance with the Medical Dictionary for Regulatory Activities.
Methods: The Japanese Adverse Drug Event Report database contained 830,079 reports published between April 2004 and April 2023. The association between herbal medicines and DILLD was evaluated using the pharmacovigilance index as the reporting odds ratio (ROR), logistic regression models, propensity score-matching techniques, and Weibull shape parameters.
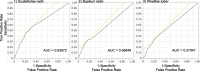
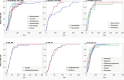
Results: The adjusted RORs using multivariate logistic regression models for Scutellariae radix (daily intake), Pinelliae tuber (daily intake), sex (male), age (≥ 60 years), Scutellariae radix (daily intake)*age (≥ 60 years), and Scutellariae radix (daily intake)* Pinelliae tuber (daily intake) were 1.47 (1.36 - 1.59), 1.05 (1.01 - 1.10), 1.45 (1.34 - 1.57), 1.92 (1.74 - 2.11), 3.35 (3.12 - 3.60), and 1.49 (1.46 - 1.53), respectively. DIILD onset profiles were evaluated using the Weibull shape parameter. A logistic plot of daily intake and onset of DIILD was drawn. ROR signals were detected in 32 of 54 herbal medicines, including Scutellariae radix, Bupleuri radix, and Pinelliae tuber. The median duration (days) (interquartile range) to DIILD onset was 36.0 (27.0-63.0) for Saikokaryukotsuboreito, 35.0 (21.0-55.0) for Saireito, and 31.0 (13.5-67.5) for Shosaikoto. The Weibull shape parameter beta (95% confidence interval) values for Saikokaryukotsuboreito, Saireito, and Shosaikoto were 1.36 (1.08-1.67), 1.36 (1.20-1.52), and 1.31 (0.98-1.68), respectively.
Conclusions: DIILD demonstrated a dose-dependent to crude drugs. Clinicians should strive for the early detection of DIILD and avoid the inadvertent administration of herbal medicines.
Keywords: Drug-induced interstitial lung disease; Herbal medicine; JADER; Japanese Adverse Drug Event Report database; Scutellariae radix.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Analysis of immune-related adverse events caused by immune checkpoint inhibitors using the Japanese Adverse Drug Event Report database.Pharmacoepidemiol Drug Saf. 2020 Oct;29(10):1279-1294. doi: 10.1002/pds.5108. Epub 2020 Sep 1. Pharmacoepidemiol Drug Saf. 2020. PMID: 32869941 Free PMC article.
-
Drug-induced interstitial lung disease caused by olaparib: three case reports and review of the Japanese Adverse Drug Event Report database and literature.BMC Pulm Med. 2023 Aug 8;23(1):289. doi: 10.1186/s12890-023-02569-3. BMC Pulm Med. 2023. PMID: 37553592 Free PMC article. Review.
-
Evaluation of potential complication of interstitial lung disease with abemaciclib and palbociclib treatments.Cancer Rep (Hoboken). 2022 Jan;5(1):e1402. doi: 10.1002/cnr2.1402. Epub 2021 May 3. Cancer Rep (Hoboken). 2022. PMID: 33939324 Free PMC article.
-
Evaluation of the Expression Profile of Antibiotic-Induced Thrombocytopenia Using the Japanese Adverse Drug Event Report Database.Int J Toxicol. 2021 Dec;40(6):542-550. doi: 10.1177/10915818211048151. Epub 2021 Oct 18. Int J Toxicol. 2021. PMID: 34658275
-
Efficacy and safety of the traditional herbal medication Chai-Ling-Tang (in China), Siryung-tang (in Republic of Korea) or Sairei-To (in Japan).J Ethnopharmacol. 2024 Jan 30;319(Pt 1):117127. doi: 10.1016/j.jep.2023.117127. Epub 2023 Sep 7. J Ethnopharmacol. 2024. PMID: 37683930 Review.
Cited by
-
Pharmacovigilance study of immunomodulatory drug-related adverse events using spontaneous reporting system databases.Int J Immunopathol Pharmacol. 2025 Jan-Dec;39:3946320251327618. doi: 10.1177/03946320251327618. Epub 2025 Apr 10. Int J Immunopathol Pharmacol. 2025. PMID: 40207612 Free PMC article.
-
Post-marketing safety profile of ganirelix in women: a 20-year pharmacovigilance analysis of global adverse drug event databases (2004-2024).BMC Pharmacol Toxicol. 2025 Apr 22;26(1):91. doi: 10.1186/s40360-025-00920-4. BMC Pharmacol Toxicol. 2025. PMID: 40264185 Free PMC article.
-
Estimating Adverse Events Associated With Herbal Medicines Using Pharmacovigilance Databases: Systematic Review and Meta-Analysis.JMIR Public Health Surveill. 2024 Aug 29;10:e63808. doi: 10.2196/63808. JMIR Public Health Surveill. 2024. PMID: 39208414 Free PMC article.
-
Organizing Pneumonia With Diffuse Alveolar Hemorrhage Induced by the Kampo Medicine Choreito.J Med Cases. 2024 Jul;15(7):120-125. doi: 10.14740/jmc4222. Epub 2024 Jun 19. J Med Cases. 2024. PMID: 38993806 Free PMC article.
-
Self-Organizing Map-Based Assessment of Immune-Related Adverse Events Caused by Immune Checkpoint Inhibitors.Cureus. 2025 Jan 2;17(1):e76813. doi: 10.7759/cureus.76813. eCollection 2025 Jan. Cureus. 2025. PMID: 39902026 Free PMC article.
References
-
- Ministry of Health, Labour and Welfare. The manual for handling disorders due to adverse drug reactions, interstitial lung disease. 2019. https://www.mhlw.go.jp/topics/2006/11/tp1122-1b.html. Accessed 15 Jun 2022.
-
- Tsutani K. Kusuri ha risuku : Kampoyaku kara seiyoyaku wo miru. Jpn J Pharmacoepidemiol. 2010;15:31.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

