SurvInt: a simple tool to obtain precise parametric survival extrapolations
- PMID: 38486175
- PMCID: PMC10938652
- DOI: 10.1186/s12911-024-02475-6
SurvInt: a simple tool to obtain precise parametric survival extrapolations
Abstract
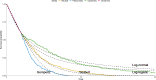
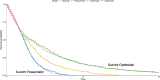
Background: Economic evaluation of emerging health technologies is mandated by agencies such as the National Institute for Health and Care Excellence (NICE) to ensure their cost is proportional to their benefit. To avoid bias, NICE stipulate that the benefit of a treatment is assessed across the lifetime of the patient population, which can be many decades. Unfortunately, follow-up from a clinical trial will not usually cover the required period and the observed follow-up will require extrapolation. For survival data this is often done by selecting a preferred model from a set of candidate parametric models. This approach is limited in that the choice of model is restricted to those originally fitted. What if none of the models are consistent with clinical prediction or external data?
Method/results: This paper introduces SurvInt, a tool that estimates the parameters of common parametric survival models which interpolate key survival time co-ordinates specified by the user, which could come from external trials, real world data or expert clinical opinion. This is achieved by solving simultaneous equations based on the survival functions of the parametric models. The application of SurvInt is shown through two examples where traditional parametric modelling did not produce models that were consistent with external data or clinical opinion. Additional features include model averaging, mixture cure models, background mortality, piecewise modelling, restricted mean survival time estimation and probabilistic sensitivity analysis.
Conclusions: SurvInt allows precise parametric survival models to be estimated and carried forward into economic models. It provides access to extrapolations that are consistent with multiple data sources such as observed data and clinical predictions, opening the door to precise exploration of regions of uncertainty/disagreement. SurvInt could avoid the need for post-hoc adjustments for complications such as treatment switching, which are often applied to obtain a plausible survival model but at the cost of introducing additional uncertainty. Phase III clinical trials are not designed with extrapolation in mind, and so it is sensible to consider alternative approaches to predict future survival that incorporate external information.
Keywords: External data; Extrapolation; Health technology assessment; Interpolation; Survival analysis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Use of Advanced Flexible Modeling Approaches for Survival Extrapolation from Early Follow-up Data in two Nivolumab Trials in Advanced NSCLC with Extended Follow-up.Med Decis Making. 2023 Jan;43(1):91-109. doi: 10.1177/0272989X221132257. Epub 2022 Oct 19. Med Decis Making. 2023. PMID: 36259353
-
Heterogeneity in Survival with Immune Checkpoint Inhibitors and Its Implications for Survival Extrapolations: A Case Study in Advanced Melanoma.MDM Policy Pract. 2022 Mar 26;7(1):23814683221089659. doi: 10.1177/23814683221089659. eCollection 2022 Jan-Jun. MDM Policy Pract. 2022. PMID: 35356551 Free PMC article.
-
General Population Mortality Adjustment in Survival Extrapolation of Cancer Trials: Exploring Plausibility and Implications for Cost-Effectiveness Analyses in HER2-Positive Breast Cancer in Sweden.Med Decis Making. 2024 Oct;44(7):843-853. doi: 10.1177/0272989X241275969. Epub 2024 Sep 12. Med Decis Making. 2024. PMID: 39263806 Free PMC article.
-
Modelling approaches for histology-independent cancer drugs to inform NICE appraisals: a systematic review and decision-framework.Health Technol Assess. 2021 Dec;25(76):1-228. doi: 10.3310/hta25760. Health Technol Assess. 2021. PMID: 34990339
-
A review and validation of overall survival extrapolation in health technology assessments of cancer immunotherapy by the National Institute for Health and Care Excellence: how did the initial best estimate compare to trial data subsequently made available?J Med Econ. 2019 Mar;22(3):205-214. doi: 10.1080/13696998.2018.1547303. Epub 2018 Nov 30. J Med Econ. 2019. PMID: 30422080 Review.
Cited by
-
Cost-effectiveness of sacituzumab tirumotecan versus chemotherapy for patients with metastatic triple-negative breast cancer in China.Breast. 2025 Aug 5;83:104550. doi: 10.1016/j.breast.2025.104550. Online ahead of print. Breast. 2025. PMID: 40768820 Free PMC article.
References
-
- NICE. Guide to the methods of technology appraisal [PMG9]. 2013.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

