A multi-country comparative study of two treponemal tests for the serodiagnosis of syphilis amongst men who have sex with men (MSM): Chemo-luminescent assay vs Treponema pallidum particle agglutination assay
- PMID: 38486194
- PMCID: PMC10941351
- DOI: 10.1186/s12879-024-09100-x
A multi-country comparative study of two treponemal tests for the serodiagnosis of syphilis amongst men who have sex with men (MSM): Chemo-luminescent assay vs Treponema pallidum particle agglutination assay
Abstract
Introduction: International guidelines recommend routine screening for syphilis (aetiological agent: Treponema pallidum subspecies pallidum) amongst key populations and vulnerable populations using tests detecting treponemal and non-treponemal antibodies. Whilst treponemal tests have high sensitivities and specificities, they differ regarding subjective or objective interpretation, throughput and workload. Chemiluminescence immunoassays (CLIAs) are cost- and time-effective automated methods for detecting treponemal antibodies. The Treponema pallidum particle agglutination assay (TPPA) has been considered the "gold standard" treponemal assay, however, this includes a highly manual procedure, low throughput and subjective interpretation. The present multi-country study evaluated the ADVIA Centaur® Syphilis CLIA (Siemens Healthcare) assay compared to the reference SERODIA-TP·PA® (Fujirebio Diagnostics) for the serodiagnosis of syphilis amongst men who have sex with men (MSM).
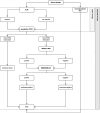
Method: 1,485 MSM were enrolled in Brighton (UK), Malta, and Verona (Italy) as part of a larger WHO multi-country and multi-site ProSPeRo study. Ethical approval was obtained. Serum was tested with the ADVIA Centaur® Syphilis CLIA assay and SERODIA-TP·PA®, in accordance with the manufacturers' instructions, for a first round of validation. A second round of validation was carried out for discrepant results that were additionally tested with both Western Blot (Westernblot EUROIMMUN®) and an Immunoblot (INNO-LIA, Fujirebio Diagnostics). Sensitivity, specificity, positive and negative predictive value (PPV and NPV), likelihood ratios (positive/negative), and the Diagnostic Odds Ratio (DOR)/pre-post-test probability (Fagan's nomogram) were calculated.
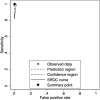
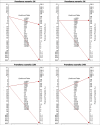
Results: Out of 1,485 eligible samples analysed in the first phase, the SERODIA-TP·PA® identified 360 positive and 1,125 negative cases. The ADVIA Centaur® Syphilis CLIA assay (Siemens) identified 366 positives, missclassifying one TPPA-positive sample. In the second phase, the ADVIA Centaur® Syphilis CLIA resulted in 1 false negative and 4 false positive results. Considering the syphilis study prevalence of 24% (95% CI: 22-26.7), The sensitivity of the ADVIA Centaur® Syphilis CLIA assay was 99.7% (95% CI: 98.5-100), and the specificity was 99.4% (95% CI: 98.7-99.7). The ROC area values were 0.996 (95% CI: 0.992-0.999), and both the PPV and NPV values were above 98% (PPV 98.1%, 95% CI: 96.1-99.2; NPV 99.9%, 95% CI: 99.5-100).
Conclusions: The ADVIA Centaur® Syphilis CLIA assay showed similar performance compared to the SERODIA-TP·PA®. Considering the study is based on QUADAS principles and with a homogeneous population, results are also likely to be generalisable to MSM population but potentially not applicable to lower prevalence populations routinely screened for syphilis. The automated CLIA treponemal assay confirmed to be accurate and appropriate for routine initial syphilis screening, i.e. when the reverse testing algorithm is applied.
Keywords: Treponema pallidum particle agglutination; Chemo-luminescent assay; MSM; Syphilis.
© 2024. The Author(s).
Conflict of interest statement
No conflict declared.
Some authors are current or former staff members of the World Health Organization. The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the views, decisions or policies of the World Health Organization.
Figures


References
-
- World Health Organization . Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021: accountability for the global health sector strategies 2016–2021. Geneva, Switzerland: World Health Organization; 2021.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

