MCT4-dependent lactate transport: a novel mechanism for cardiac energy metabolism injury and inflammation in type 2 diabetes mellitus
- PMID: 38486199
- PMCID: PMC10941417
- DOI: 10.1186/s12933-024-02178-2
MCT4-dependent lactate transport: a novel mechanism for cardiac energy metabolism injury and inflammation in type 2 diabetes mellitus
Abstract
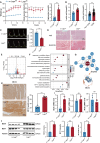
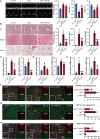
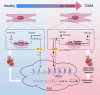
Diabetic cardiomyopathy (DCM) is a major contributor to mortality in diabetic patients, characterized by a multifaceted pathogenesis and limited therapeutic options. While lactate, a byproduct of glycolysis, is known to be significantly elevated in type 2 diabetes, its specific role in DCM remains uncertain. This study reveals an abnormal upregulation of monocarboxylate transporter 4 (MCT4) on the plasma membrane of cardiomyocytes in type 2 diabetes, leading to excessive lactate efflux from these cells. The disruption in lactate transport homeostasis perturbs the intracellular lactate-pyruvate balance in cardiomyocytes, resulting in oxidative stress and inflammatory responses that exacerbate myocardial damage. Additionally, our findings suggest increased lactate efflux augments histone H4K12 lactylation in macrophages, facilitating inflammatory infiltration within the microenvironment. In vivo experiments have demonstrated that inhibiting MCT4 effectively alleviates myocardial oxidative stress and pathological damage, reduces inflammatory macrophage infiltration, and enhances cardiac function in type 2 diabetic mice. Furthermore, a clinical prediction model has been established, demonstrating a notable association between peripheral blood lactate levels and diastolic dysfunction in individuals with type 2 diabetes. This underscores the potential of lactate as a prognostic biomarker for DCM. Ultimately, our findings highlight the pivotal involvement of MCT4 in the dysregulation of cardiac energy metabolism and macrophage-mediated inflammation in type 2 diabetes. These insights offer novel perspectives on the pathogenesis of DCM and pave the way for the development of targeted therapeutic strategies against this debilitating condition.
Keywords: Diabetic cardiomyopathy; Inflammation; Lactate; Lipotoxicity; MCT4.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

