Autotaxin inhibition attenuates the aortic valve calcification by suppressing inflammation-driven fibro-calcific remodeling of valvular interstitial cells
- PMID: 38486246
- PMCID: PMC10941471
- DOI: 10.1186/s12916-024-03342-x
Autotaxin inhibition attenuates the aortic valve calcification by suppressing inflammation-driven fibro-calcific remodeling of valvular interstitial cells
Erratum in
-
Correction: Autotaxin inhibition attenuates the aortic valve calcification by suppressing inflammation-driven fibro-calcific remodeling of valvular interstitial cells.BMC Med. 2024 Apr 29;22(1):178. doi: 10.1186/s12916-024-03403-1. BMC Med. 2024. PMID: 38679711 Free PMC article. No abstract available.
Abstract
Background: Patients with fibro-calcific aortic valve disease (FCAVD) have lipid depositions in their aortic valve that engender a proinflammatory impetus toward fibrosis and calcification and ultimately valve leaflet stenosis. Although the lipoprotein(a)-autotaxin (ATX)-lysophosphatidic acid axis has been suggested as a potential therapeutic target to prevent the development of FCAVD, supportive evidence using ATX inhibitors is lacking. We here evaluated the therapeutic potency of an ATX inhibitor to attenuate valvular calcification in the FCAVD animal models.
Methods: ATX level and activity in healthy participants and patients with FCAVD were analyzed using a bioinformatics approach using the Gene Expression Omnibus datasets, enzyme-linked immunosorbent assay (ELISA), immunohistochemistry, and western blotting. To evaluate the efficacy of ATX inhibitor, interleukin-1 receptor antagonist-deficient (Il1rn-/-) mice and cholesterol-enriched diet-induced rabbits were used as the FCAVD models, and primary human valvular interstitial cells (VICs) from patients with calcification were employed.
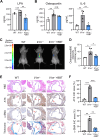
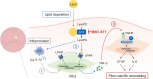
Results: The global gene expression profiles of the aortic valve tissue of patients with severe FCAVD demonstrated that ATX gene expression was significantly upregulated and correlated with lipid retention (r = 0.96) or fibro-calcific remodeling-related genes (r = 0.77) in comparison to age-matched non-FCAVD controls. Orally available ATX inhibitor, BBT-877, markedly ameliorated the osteogenic differentiation and further mineralization of primary human VICs in vitro. Additionally, ATX inhibition significantly attenuated fibrosis-related factors' production, with a detectable reduction of osteogenesis-related factors, in human VICs. Mechanistically, ATX inhibitor prohibited fibrotic changes in human VICs via both canonical and non-canonical TGF-β signaling, and subsequent induction of CTGF, a key factor in tissue fibrosis. In the in vivo FCAVD model system, ATX inhibitor exposure markedly reduced calcific lesion formation in interleukin-1 receptor antagonist-deficient mice (Il1rn-/-, P = 0.0210). This inhibition ameliorated the rate of change in the aortic valve area (P = 0.0287) and mean pressure gradient (P = 0.0249) in the FCAVD rabbit model. Moreover, transaortic maximal velocity (Vmax) was diminished with ATX inhibitor administration (mean Vmax = 1.082) compared to vehicle control (mean Vmax = 1.508, P = 0.0221). Importantly, ATX inhibitor administration suppressed the effects of a high-cholesterol diet and vitamin D2-driven fibrosis, in association with a reduction in macrophage infiltration and calcific deposition, in the aortic valves of this rabbit model.
Conclusions: ATX inhibition attenuates the development of FCAVD while protecting against fibrosis and calcification in VICs, suggesting the potential of using ATX inhibitors to treat FCAVD.
Keywords: Aortic valve; Autotaxin; Calcification; Fibro-calcific aortic valve disease; Fibrosis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing financial or other interests in relation to this study.
Figures





Similar articles
-
Evogliptin Suppresses Calcific Aortic Valve Disease by Attenuating Inflammation, Fibrosis, and Calcification.Cells. 2021 Jan 1;10(1):57. doi: 10.3390/cells10010057. Cells. 2021. PMID: 33401457 Free PMC article.
-
Dipeptidyl Peptidase-4 Induces Aortic Valve Calcification by Inhibiting Insulin-Like Growth Factor-1 Signaling in Valvular Interstitial Cells.Circulation. 2017 May 16;135(20):1935-1950. doi: 10.1161/CIRCULATIONAHA.116.024270. Epub 2017 Feb 8. Circulation. 2017. PMID: 28179397
-
Transforming growth factor-β1 promotes fibrosis but attenuates calcification of valvular tissue applied as a three-dimensional calcific aortic valve disease model.Am J Physiol Heart Circ Physiol. 2020 Nov 1;319(5):H1123-H1141. doi: 10.1152/ajpheart.00651.2019. Epub 2020 Sep 28. Am J Physiol Heart Circ Physiol. 2020. PMID: 32986963
-
Fibrocalcific aortic valve disease: opportunity to understand disease mechanisms using mouse models.Circ Res. 2013 Jul 5;113(2):209-22. doi: 10.1161/CIRCRESAHA.113.300153. Circ Res. 2013. PMID: 23833295 Free PMC article. Review.
-
Autotaxin and Lipoprotein Metabolism in Calcific Aortic Valve Disease.Front Cardiovasc Med. 2019 Mar 1;6:18. doi: 10.3389/fcvm.2019.00018. eCollection 2019. Front Cardiovasc Med. 2019. PMID: 30881959 Free PMC article. Review.
Cited by
-
The Combined Effect of the Systemic Immune-Inflammation Index and Aortic Valve Calcification on Major Adverse Cardiovascular Events in Patients with Coronary Heart Disease.J Inflamm Res. 2024 Nov 7;17:8375-8384. doi: 10.2147/JIR.S493735. eCollection 2024. J Inflamm Res. 2024. PMID: 39529998 Free PMC article.
-
Ex vivo model of pathological calcification of human aortic valve.Front Cardiovasc Med. 2024 Aug 29;11:1411398. doi: 10.3389/fcvm.2024.1411398. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 39280032 Free PMC article.
-
Correction: Autotaxin inhibition attenuates the aortic valve calcification by suppressing inflammation-driven fibro-calcific remodeling of valvular interstitial cells.BMC Med. 2024 Apr 29;22(1):178. doi: 10.1186/s12916-024-03403-1. BMC Med. 2024. PMID: 38679711 Free PMC article. No abstract available.
-
Myeloid-specific deletion of autotaxin inhibits rheumatoid arthritis and osteoclastogenesis.Front Immunol. 2024 Dec 18;15:1481699. doi: 10.3389/fimmu.2024.1481699. eCollection 2024. Front Immunol. 2024. PMID: 39744622 Free PMC article.
-
Biomarkers of Calcification, Endothelial Injury, and Platelet-Endothelial Interaction in Patients with Aortic Valve Stenosis.Int J Mol Sci. 2025 May 19;26(10):4873. doi: 10.3390/ijms26104873. Int J Mol Sci. 2025. PMID: 40430015 Free PMC article.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

