Efficacy and safety of follitropin delta for ovarian stimulation in vitro fertilization/ intracytoplasmic sperm injection cycles: a systematic review with meta-analysis
- PMID: 38486276
- PMCID: PMC10938807
- DOI: 10.1186/s13048-024-01372-w
Efficacy and safety of follitropin delta for ovarian stimulation in vitro fertilization/ intracytoplasmic sperm injection cycles: a systematic review with meta-analysis
Abstract
Background: Follitropin delta is a novel recombinant follicle stimulating hormone preparation uniquely expressed in a human fetal retinal cell line by recombinant DNA technology. To date, no systematic review was available about the safety and the efficacy of the follitropin delta. The objective of this study was systematically reviewing the available literature and to provide updated evidence regarding the efficacy-safety profile of follitropin delta when compared to other gonadotropin formulations for ovarian stimulation in in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) cycles.
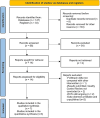
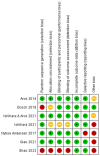
Methods: An extensive search was performed to identify phase 1, phase 2 and phase 3 RCTs in humans focused on follitropin delta use for ovarian stimulation in IVF/ICSI cycles. The risk of bias and the overall quality of the evidence was analyzed. All data were extracted and analyzed using the intention-to-treat principle and expressed per woman randomized.
Results: A total of 7 RCTs (1 phase 1 RCT, 2 phase 2 RCTs and 4 phase 3 RCTs) were included in the qualitative analysis, whereas data of three phase 3 RCTs were meta-analyzed. All trials compared personalized recombinant follitropin delta treatment versus conventional recombinant follitropin alfa/beta administration in potentially normo-responder patients who receive ovarian stimulation in GnRH antagonist IVF/ICSI cycles. No difference between two regimens was detected for clinical pregnancy rate [odds ratio (OR) 1.06; 95% confidence intervals (CI): 0.90, 1.24; P = 0.49; I2 = 26%], ongoing pregnancy rate (OR 1.15; 95%CI: 0.90, 1.46; P = 0.27; I2 = 40%), and live birth rate (OR 1.18; 95%CI: 0.89, 1.55; P = 0.25; I2 = 55%). No data were available regarding cumulative success rates. The rate of adoption of strategies to prevent ovarian hyperstimulation syndrome (OHSS) development (OR 0.45; 95%CI: 0.30, 0.66; P < 0.0001; I2 = 0%), and the rate of both early OHSS (OR 0.62; 95%CI: 0.43, 0.88; P = 0.008; I2 = 0%) and all forms of OHSS (OR 0.61; 95%CI: 0.44, 0.84; P = 0.003; I2 = 0%) were significantly lower in the group of patients treated with personalized follitropin delta treatment compared to those treated with conventional follitropin alfa/beta administration.
Conclusion: Personalized follitropin delta treatment is associated with a lower risk of OHSS compared to conventional follitropin alfa/beta administration in potentially normo-responder patients who receive ovarian stimulation in GnRH antagonist IVF/ICSI cycles. The absence of cumulative data does not allow definitive conclusions to be drawn regarding the comparison of the effectiveness of the two treatments.
Protocol study registration: CRD42023470352 (available at http://www.crd.york.ac.uk/PROSPERO ).
Keywords: Follitropin delta; Gonadotropin; IVF; Infertility; Ovarian stimulation; Sterility.
© 2024. The Author(s).
Conflict of interest statement
SP, DC, PELS, and AB have no conflict of interest to declare.
Figures

References
-
- European IVF, Monitoring Consortium (EIM), for the European Society of Human Reproduction and Embryology (ESHRE), Wyns C, De Geyter C, Calhaz-Jorge C, Kupka MS, Motrenko T, Smeenk J, Bergh C, Tandler-Schneider A, Rugescu IA. Goossens V. ART in Europe, 2018: results generated from European registries by ESHRE. Hum Reprod Open. 2022; 2022:hoac022. 10.1093/hropen/hoac022. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

