Impact of plasma Epstein-Barr virus DNA in posttreatment nasopharyngeal carcinoma patients after SARS-CoV-2 infection
- PMID: 38486290
- PMCID: PMC10938826
- DOI: 10.1186/s13027-024-00570-x
Impact of plasma Epstein-Barr virus DNA in posttreatment nasopharyngeal carcinoma patients after SARS-CoV-2 infection
Abstract
Background: Nasopharyngeal carcinoma (NPC) is prevalent in southern China. EBV DNA is the most useful biomarker in NPC. However, the value of EBV DNA in posttreatment NPC patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) remains unclear.
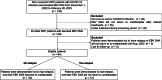
Methods: Sixty-four eligible NPC patients were enrolled between December 2022 and February 2023. Patients who met the following criteria were included: had non-metastatic NPC, completed radical treatment, were first firstly infected with SARS-CoV-2 and their EBV DNA changed from undetectable to detectable.
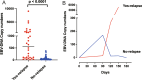
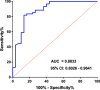
Results: At the end of follow-up, 81.25% (52/64) of patients were confirmed not to relapse with undetectable EBV DNA (no-relapse). In addition, 18.75% (12/64) of patients experienced relapse with consistent detection of EBV DNA (yes-relapse). For all 64 patients, the average time from diagnosis of coronavirus disease 2019 (COVID-19) to detection of detectable EBV DNA was 35.41 days (2 to 139 days). For 52 no-relapse patients, the average time from EBV DNA changing from detectable to undetectable was 63.12 days (6 to 147 days). The levels of EBV DNA were greater in yes-relapse patients than that in no-relapse patients, and the average of EBV DNA levels were 1216 copies/ml and 53.18 copies/ml, respectively. Using 62.3 copies/mL as the threshold, the area under the curve for EBV DNA was 0.88 for distinguishing yes-relapse patients from no-relapse patients. The sensitivity and specificity were 81.97% (95% CI 0.71-0.95) and 86.67% (95% CI 0.70-0.95), respectively.
Conclusion: For NPC patients infected with SARS-CoV-2, EBV DNA alone is insufficient for monitoring relapse after radical therapy. Long-term follow-up and underlying mechanistic investigations of EBV DNA changes are urgently needed.
Keywords: COVID-19; EBV DNA; Nasopharyngeal carcinoma; Omicron; Relapse.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Prognostic value of plasma Epstein-Barr virus DNA level during posttreatment follow-up in the patients with nasopharyngeal carcinoma having undergone intensity-modulated radiotherapy.Chin J Cancer. 2017 Nov 7;36(1):87. doi: 10.1186/s40880-017-0256-x. Chin J Cancer. 2017. PMID: 29116021 Free PMC article.
-
Long-term prognostic effects of plasma epstein-barr virus DNA by minor groove binder-probe real-time quantitative PCR on nasopharyngeal carcinoma patients receiving concurrent chemoradiotherapy.Int J Radiat Oncol Biol Phys. 2007 Aug 1;68(5):1342-8. doi: 10.1016/j.ijrobp.2007.02.012. Epub 2007 Apr 20. Int J Radiat Oncol Biol Phys. 2007. PMID: 17449194 Clinical Trial.
-
Long-term monitoring of dynamic changes in plasma EBV DNA for improved prognosis prediction of nasopharyngeal carcinoma.Cancer Med. 2021 Feb;10(3):883-894. doi: 10.1002/cam4.3669. Epub 2020 Dec 30. Cancer Med. 2021. PMID: 33378109 Free PMC article.
-
A systematic review and recommendations on the use of plasma EBV DNA for nasopharyngeal carcinoma.Eur J Cancer. 2021 Aug;153:109-122. doi: 10.1016/j.ejca.2021.05.022. Epub 2021 Jun 18. Eur J Cancer. 2021. PMID: 34153713
-
Clinical utility of Epstein-Barr virus DNA and other liquid biopsy markers in nasopharyngeal carcinoma.Cancer Commun (Lond). 2020 Nov;40(11):564-585. doi: 10.1002/cac2.12100. Epub 2020 Sep 28. Cancer Commun (Lond). 2020. PMID: 32989921 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

