Incidence and Predictors of Tuberculosis-associated IRIS in People With HIV Treated for Tuberculosis: Findings From Reflate TB2 Randomized Trial
- PMID: 38486816
- PMCID: PMC10939434
- DOI: 10.1093/ofid/ofae035
Incidence and Predictors of Tuberculosis-associated IRIS in People With HIV Treated for Tuberculosis: Findings From Reflate TB2 Randomized Trial
Abstract
Background: After antiretroviral therapy (ART) initiation, people with HIV (PWH) treated for tuberculosis (TB) may develop TB-associated immune reconstitution inflammatory syndrome (TB-IRIS). Integrase inhibitors, by providing a faster HIV-RNA decline than efavirenz, might increase the risk for this complication. We sought to assess incidence and determinants of TB-IRIS in PWH with TB on raltegravir- or efavirenz-based ART.
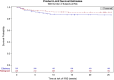
Methods: We conducted a secondary analysis of the Reflate TB 2 trial, which randomized ART-naive PWH on standard TB treatment, to receive raltegravir- or efavirenz-based ART. The primary objective was to evaluate the incidence of TB-IRIS. Incidence rate ratio comparing TB-IRIS incidence in each arm was calculated. Kaplan-Meier curves were used to compare TB-IRIS-free survival probabilities by ART arm. Cox regression models were fitted to analyze baseline characteristics associated with TB-IRIS.
Results: Of 460 trial participants, 453 from Brazil, Côte d'Ivoire, Mozambique, and Vietnam were included in this analysis. Baseline characteristics were median age 35 years (interquartile range [IQR], 29-43), 40% female, 69% pulmonary TB only, median CD4, 102 (IQR, 38-239) cells/mm³, and median HIV RNA, 5.5 (IQR, 5.0-5.8) log copies/mL. Forty-eight participants developed TB-IRIS (incidence rate, 24.7/100 PY), 19 cases in the raltegravir arm and 29 in the efavirenz arm (incidence rate ratio 0.62, 95% confidence interval .35-1.10). Factors associated with TB-IRIS were: CD4 ≤ 100 cells/μL, HIV RNA ≥500 000 copies/mL, and extrapulmonary/disseminated TB.
Conclusions: We did not demonstrate that raltegravir-based ART increased the incidence of TB-IRIS compared with efavirenz-based ART. Low CD4 counts, high HIV RNA, and extrapulmonary/disseminated TB at ART initiation were associated with TB-IRIS.
Keywords: HIV/AIDS; IRIS; antiretroviral therapy; randomized controlled trial; tuberculosis.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. J. M. M. has acted as a consultant, participated in advisory boards, has received speaker fees, and has been an investigator for clinical trials for ViiV Healthcare, Gilead Sciences, and Merck. He has also received research grants from Gilead Sciences. C. D. participated in advisory boards for ViiV Healthcare, Gilead Sciences, BMS, and Merck, and has also received research grants from Gilead and MAD. All other authors report no potential conflicts.
Figures
References
-
- Global Tuberculosis Report 2022 . 2023. Available at: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/globa.... - PubMed
-
- World Health Organization . Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. Available at: https://apps.who.int/iris/handle/10665/342899. - PubMed
-
- Dutertre M, Cuzin L, Demonchy E, et al. Initiation of antiretroviral therapy containing integrase inhibitors increases the risk of IRIS requiring hospitalization. J Acquir Immune Defic Syndr 2017; 76:e23–6. - PubMed
-
- Namale PE, Abdullahi LH, Fine S, Kamkuemah M, Wilkinson RJ, Meintjes G. Paradoxical TB-IRIS in HIV-infected adults: a systematic review and meta-analysis. Future Microbiol 2015; 10:1077–99. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

