PyF2F: a robust and simplified fluorophore-to-fluorophore distance measurement tool for Protein interactions from Imaging Complexes after Translocation experiments
- PMID: 38486885
- PMCID: PMC10939353
- DOI: 10.1093/nargab/lqae027
PyF2F: a robust and simplified fluorophore-to-fluorophore distance measurement tool for Protein interactions from Imaging Complexes after Translocation experiments
Abstract
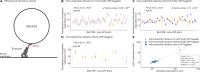
Structural knowledge of protein assemblies in their physiological environment is paramount to understand cellular functions at the molecular level. Protein interactions from Imaging Complexes after Translocation (PICT) is a live-cell imaging technique for the structural characterization of macromolecular assemblies in living cells. PICT relies on the measurement of the separation between labelled molecules using fluorescence microscopy and cell engineering. Unfortunately, the required computational tools to extract molecular distances involve a variety of sophisticated software programs that challenge reproducibility and limit their implementation to highly specialized researchers. Here we introduce PyF2F, a Python-based software that provides a workflow for measuring molecular distances from PICT data, with minimal user programming expertise. We used a published dataset to validate PyF2F's performance.
© The Author(s) 2024. Published by Oxford University Press on behalf of NAR Genomics and Bioinformatics.
Figures


References
-
- Yildiz A., Selvin P.R. Fluorescence imaging with one nanometer accuracy: application to molecular motors. Acc. Chem. Res. 2005; 38:574–582. - PubMed
LinkOut - more resources
Full Text Sources

