Longitudinal rheumatoid factor autoantibody responses after SARS-CoV-2 vaccination or infection
- PMID: 38487524
- PMCID: PMC10937420
- DOI: 10.3389/fimmu.2024.1314507
Longitudinal rheumatoid factor autoantibody responses after SARS-CoV-2 vaccination or infection
Abstract
Background: Rheumatoid factors (RFs) are autoantibodies that target the Fc region of IgG, and are found in patients with rheumatic diseases as well as in the healthy population. Many studies suggest that an immune trigger may (transiently) elicit RF responses. However, discrepancies between different studies make it difficult to determine if and to which degree RF reactivity can be triggered by vaccination or infection.
Objective: We quantitatively explored longitudinal RF responses after SARS-CoV-2 vaccination and infection in a well-defined, large cohort using a dual ELISA method that differentiates between true RF reactivity and background IgM reactivity. In addition, we reviewed existing literature on RF responses after vaccination and infection.
Methods: 151 healthy participants and 30 RA patients were included to measure IgM-RF reactivity before and after SARS-CoV-2 vaccinations by ELISA. Additionally, IgM-RF responses after a SARS-CoV-2 breakthrough infection were studied in 51 healthy participants.
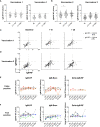
Results: Published prevalence studies in subjects after infection report up to 85% IgM-RF seropositivity. However, seroconversion studies (both infection and vaccination) report much lower incidences of 2-33%, with a trend of lower percentages observed in larger studies. In the current study, SARS-CoV-2 vaccination triggered low-level IgM-RF responses in 5.5% (8/151) of cases, of which 1.5% (2/151) with a level above 10 AU/mL. Breakthrough infection was accompanied by development of an IgM-RF response in 2% (1/51) of cases.
Conclusion: Our study indicates that de novo RF induction following vaccination or infection is an uncommon event, which does not lead to RF epitope spreading.
Keywords: SARS-CoV-2; autoantibodies; autoimmunity; infection; rheumatoid arthritis; rheumatoid factor; vaccination.
Copyright © 2024 Keijzer, Oskam, Ooijevaar-de Heer, Steenhuis, Keijser, Wieske, van Dam, Stalman, Kummer, Boekel, Kuijpers, ten Brinke, van Ham, Eftimov, Tas, Wolbink and Rispens.
Conflict of interest statement
TR and GW are inventors on a patent application based on the use of bioengineered IgG targets for the characterization of rheumatoid factor reactivity patterns. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Sobhy N, Ghoniem SA, Eissa BM, Kamal A, Medhat A, Elsaid NY. Disease characteristics in high versus low titers of rheumatoid factor or anti-citrullinated peptide antibody in rheumatoid arthritis patients. Egypt Rheumatol. (2022) 44:325–8. doi: 10.1016/j.ejr.2022.04.004 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

