SKAP2 acts downstream of CD11b/CD18 and regulates neutrophil effector function
- PMID: 38487529
- PMCID: PMC10937362
- DOI: 10.3389/fimmu.2024.1344761
SKAP2 acts downstream of CD11b/CD18 and regulates neutrophil effector function
Abstract
Background: The importance of CD11b/CD18 expression in neutrophil effector functions is well known. Beyond KINDLIN3 and TALIN1, which are involved in the induction of the high-affinity binding CD11b/CD18 conformation, the signaling pathways that orchestrate this response remain incompletely understood.
Method: We performed an unbiased screening method for protein selection by biotin identification (BioID) and investigated the KINDLIN3 interactome. We used liquid chromatography with tandem mass spectrometry as a powerful analytical tool. Generation of NB4 CD18, KINDLIN3, or SKAP2 knockout neutrophils was achieved using CRISPR-Cas9 technology, and the cells were examined for their effector function using flow cytometry, live cell imaging, microscopy, adhesion, or antibody-dependent cellular cytotoxicity (ADCC).
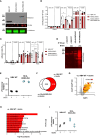
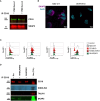
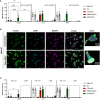
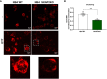
Results: Among the 325 proteins significantly enriched, we identified Src kinase-associated phosphoprotein 2 (SKAP2), a protein involved in actin polymerization and integrin-mediated outside-in signaling. CD18 immunoprecipitation in primary or NB4 neutrophils demonstrated the presence of SKAP2 in the CD11b/CD18 complex at a steady state. Under this condition, adhesion to plastic, ICAM-1, or fibronectin was observed in the absence of SKAP2, which could be abrogated by blocking the actin rearrangements with latrunculin B. Upon stimulation of NB4 SKAP2-deficient neutrophils, adhesion to fibronectin was enhanced whereas CD18 clustering was strongly reduced. This response corresponded with significantly impaired CD11b/CD18-dependent NADPH oxidase activity, phagocytosis, and cytotoxicity against tumor cells.
Conclusion: Our results suggest that SKAP2 has a dual role. It may restrict CD11b/CD18-mediated adhesion only under resting conditions, but its major contribution lies in the regulation of dynamic CD11b/CD18-mediated actin rearrangements and clustering as required for cellular effector functions of human neutrophils.
Keywords: CD11b/CD18 integrin; Src kinase associated phosphoprotein 2 (SKAP2); adhesion; antibody-dependent cellular cytotoxicity (ADCC); filamentous actin; neutrophils; phagocytosis.
Copyright © 2024 Bouti, Klein, Verkuijlen, Schornagel, van Alphen, Taris, van den Biggelaar, Hoogendijk, van Bruggen, Kuijpers and Matlung.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

