Okapi-EM: A napari plugin for processing and analyzing cryogenic serial focused ion beam/scanning electron microscopy images
- PMID: 38487692
- PMCID: PMC10936406
- DOI: 10.1017/S2633903X23000119
Okapi-EM: A napari plugin for processing and analyzing cryogenic serial focused ion beam/scanning electron microscopy images
Abstract
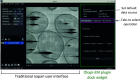
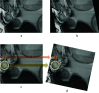
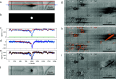
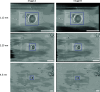
An emergent volume electron microscopy technique called cryogenic serial plasma focused ion beam milling scanning electron microscopy (pFIB/SEM) can decipher complex biological structures by building a three-dimensional picture of biological samples at mesoscale resolution. This is achieved by collecting consecutive SEM images after successive rounds of FIB milling that expose a new surface after each milling step. Due to instrumental limitations, some image processing is necessary before 3D visualization and analysis of the data is possible. SEM images are affected by noise, drift, and charging effects, that can make precise 3D reconstruction of biological features difficult. This article presents Okapi-EM, an open-source napari plugin developed to process and analyze cryogenic serial pFIB/SEM images. Okapi-EM enables automated image registration of slices, evaluation of image quality metrics specific to pFIB-SEM imaging, and mitigation of charging artifacts. Implementation of Okapi-EM within the napari framework ensures that the tools are both user- and developer-friendly, through provision of a graphical user interface and access to Python programming.
Keywords: 3D imaging; Cryogenic; image processing; napari; serial FIB/SEM; software.
© The Author(s) 2023.
Conflict of interest statement
M.C.D. is an employee of SPT Labtech. The remaining authors declare that they have no competing interests.
Figures









References
-
- Dumoux M, Glen T, Ho EML, et al. (2022) Cryo-plasma FIB/SEM volume imaging of biological specimens, p. 2022.09.21.508877. https://www.biorxiv.org/content/10.1101/2022.09.21.508877v1 (accessed October 25, 2022).
-
- Vidavsky N, Akiva A, Kaplan-Ashiri I, et al. (2016) Cryo-FIB-SEM serial milling and block face imaging: large volume structural analysis of biological tissues preserved close to their native state. J Struct Biol 196, 487–495. - PubMed
-
- Spehner D, Steyer AM, Bertinetti L, et al. (2020) Cryo-FIB-SEM as a promising tool for localizing proteins in 3D. J Struct Biol 211, 107528. - PubMed
-
- Kilpatrick PK, Miller WG & Talmon Y (1985) Staining and drying-induced artifacts in electron microscopy of surfactant dispersions. II. Change in phase behavior produced by variation in ph modifiers, stain, and concentration. J Colloid Interface Sci 107, 146–158.
LinkOut - more resources
Full Text Sources
